Methyl anisoles
| Methyl anisoles | |||||||
| Surname | 2-methyl anisole | 3-methyl anisole | 4-methyl anisole | ||||
| other names |
o -Methylanisol, 2-methoxytoluene, o -Kresylmethylether |
m -Methylanisol, 3-methoxytoluene, m -Kresylmethylether |
p -Methylanisol, 4-methoxytoluene, p -Kresylmethylether |
||||
| Structural formula |
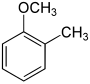
|

|

|
||||
| CAS number | 578-58-5 | 100-84-5 | 104-93-8 | ||||
| ECHA InfoCard | 100.008.571 | 100.002.631 | 100.002.958 | ||||
| PubChem | 33637 | 7530 | 7731 | ||||
| Molecular formula | C 8 H 10 O | ||||||
| Molar mass | 122.17 g mol −1 | ||||||
| Physical state | liquid | ||||||
| Brief description | colorless liquid | yellowish liquid | yellowish liquid | ||||
| Melting point | −34.1 ° C | - | −50 ° C | ||||
| boiling point | 170-172 ° C | 175-180 ° C | 174-177 ° C | ||||
|
GHS labeling |
|
|
|
||||
| H and P phrases | 226 | 226 | 226-302-315 | ||||
| no EUH phrases | no EUH phrases | no EUH phrases | |||||
| no P-phrases | no P-phrases | no P-phrases | |||||
The methyl anisoles (also methoxytoluenes or cresyl methyl ether ) form a group of substances in chemistry that is derived from both anisole and toluene . The structure consists of a benzene ring with attached methoxy (–OCH 3 ) and methyl groups (–CH 3 ) as substituents . Their different arrangement results in three constitutional isomers with the empirical formula C 8 H 10 O.
properties
The methyl anisoles are colorless to yellowish liquids that boil in the range from 170 to 180 ° C.
presentation
Methyl anisoles can be prepared from the cresols by etherification with dimethyl sulfate .
Risk assessment
In 2012, 4-methyl anisole was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. Ingestion of 4-methyl anisole was caused by concerns about its classification as a CMR substance, consumer use , high risk characterization ratio (RCR) and widespread use. The re-evaluation took place from 2012 and was carried out by Ireland . A final report was then published.
Web links
Individual evidence
- ↑ a b c Entry on 2-methyl anisole in the GESTIS substance database of the IFA , accessed on March 13, 2017(JavaScript required) .
- ↑ a b c Entry on 3-methyl anisole in the GESTIS substance database of the IFA , accessed on March 13, 2017(JavaScript required) .
- ↑ a b c Entry on 4-methyl anisole in the GESTIS substance database of the IFA , accessed on March 13, 2017(JavaScript required) .
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 209.
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): 4-methylanisole , accessed on May 20, 2019.

