Phenetol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
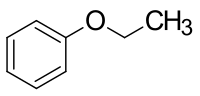
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Phenetol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 10 O | ||||||||||||||||||
| Brief description |
colorless to yellowish, oily liquid with an aromatic odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 122.17 g · mol -1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.97 g cm −3 |
||||||||||||||||||
| Melting point |
−30 ° C |
||||||||||||||||||
| boiling point |
|
||||||||||||||||||
| solubility |
practically insoluble in water (0.57 g l −1 at 25 ° C) |
||||||||||||||||||
| Refractive index |
1.507 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Phenetol is an organic chemical compound and a pleasant smelling, colorless liquid. Since phenetol can formally be understood as an ether made from phenol and ethanol , it is also known as ethylphenyl ether or ethoxybenzene . The latter is the systematic name of this compound.
presentation
On a laboratory scale, phenetole can be obtained by the etherification of phenol, e.g. B. by reaction with diethyl sulfate in the presence of a base .
use
Phenetol is used as a special solvent in the laboratory and in numerous organic syntheses.
Individual evidence
- ↑ a b c d e f g Entry on phenetol in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 340, ISBN 3-342-00280-8 .
- ↑ Data sheet Ethoxybenzene from Sigma-Aldrich , accessed on May 29, 2011 ( PDF ).
- ↑ Hans-Dieter Jakubke, Ruth Karcher (coordinators): Encyclopedia of chemistry in three volumes, Spektrum Verlag, Heidelberg, Volume 3, 1999, ISBN 3-8274-0381-2 , pp 13-14.
