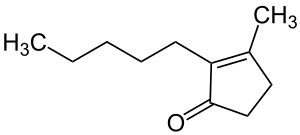
Dihydrojasmone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Dihydrojasmone | ||||||||||||||||||
| other names |
2-pentyl-3-methyl-2-cyclopenten-1-one |
||||||||||||||||||
| Molecular formula | C 11 H 18 O | ||||||||||||||||||
| Brief description |
colorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 166.26 g · mol -1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.916 g cm −3 (25 ° C) |
||||||||||||||||||
| boiling point |
120-121 ° C (12 mmHg) |
||||||||||||||||||
| solubility |
almost insoluble in water, soluble in ethanol |
||||||||||||||||||
| Refractive index |
1.4767 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Dihydrojasmone is one of jasmone derived chemical compound . It is a colorless liquid with a characteristic smell that is used in perfumes as an odorous substance (jasmine-like).
presentation
The synthesis of dihydrojasmone was first described by Heinz Hunsdiecker in 1942 . In the Hunsdiecker condensation , 2,5-undecanedione is cyclized in boiling ethanol with 2 percent aqueous sodium hydroxide solution as a catalyst .
2,5-Undecanedione can be obtained by cleaving 2-hexyl- 5-methylfuran , which can be obtained from 5-methylfurfural in a multi-stage reaction sequence with poor yield . An alternative process for the production of 2,5-undecanedione starts from n - heptanal , whereby the carbonyl group is protected with 1,3-propanedithiol as dithiane . After alkylation and cleavage of the protective group , the starting compound for the dihydrojasmone synthesis is obtained.
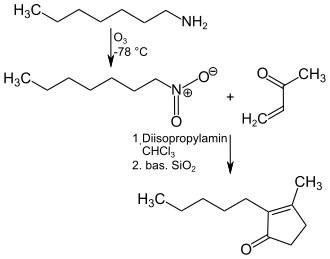
A technically simple synthesis of dihydrojasmone starts from n- heptylamine , which is oxidized with ozone at −78 ° C to 1-nitroheptane . This is dissolved in chloroform and reacted with catalytic amounts of diisopropylamine at 40 ° C. with methyl vinyl ketone and then evaporated to dryness with basic silica gel and converted to the end product at 80 ° C. for 48 hours.
Alternative synthesis routes use 2-methylfuran or methyl acrylate as the starting component .
Individual evidence
- ↑ a b c d e f Data sheet Dihydrojasmone, ≥98%, stabilized, FG from Sigma-Aldrich , accessed on December 26, 2019 ( PDF ).
- ↑ data sheet at TheGoodScents
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-366.
- ↑ a b Heinz Hunsdiecker: About the behavior of γ-diketones, 1st communication . In: Reports of the German Chemical Society . tape 75 , no. 5 , May 6, 1942, pp. 447-454 , doi : 10.1002 / cber.19420750502 .
- ↑ Patent US2387587 : Process of manufacturing cyclopentenone derivatives. Registered on August 20, 1940 , published October 23, 1945 , inventor: Heinz Hunsdiecker.
- ↑ External identifiers or database links for 5-methylfurfural : CAS number: 620-02-0, EC number: 210-622-6, ECHA InfoCard: 100.009.658 , GESTIS substance database : 493494 , PubChem : 12097 , ChemSpider : 11600 , Wikidata : Q22830264 .
- ↑ Tse-Lok Ho, Honor C. Ho, CM Wong: A Synthetic Route to Dihydrojasmone; Sulfuric Acid as Dethioacetalization Agent . In: Canadian Journal of Chemistry . 51 (2), 1973, pp. 153-155, doi : 10.1139 / v73-023 .
- ^ Ehud Keinan, Yehuda Mazur: Reactions in dry media. A simple conversion of nitro groups into carbonyls . In: Journal of the American Chemical Society . tape 99 , no. March 11 , 1977, p. 3861 , doi : 10.1021 / ja00453a067 .
- ↑ Janusz Nowicki: Review: Synthesis of Jasmonoides from Furan Derivatives , in: Molecules , 2000 , 5 , pp. 1201-1209.
- ^ GW Parshall, WA Nugent, DM-T. Chan, W. Tam: A new role for organometallic reactions in organic synthesis in industry (PDF; 220 kB) , in: Pure Appl. Chem. , 1985 , 57 (12), pp. 1809-1818.