Diisopropylamine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
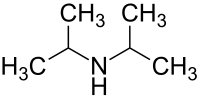
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Diisopropylamine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 15 N | |||||||||||||||
| Brief description |
highly volatile, highly flammable, colorless liquid with an amine-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 101.19 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.72 g cm −3 |
|||||||||||||||
| Melting point |
−61 ° C |
|||||||||||||||
| boiling point |
82 ° C |
|||||||||||||||
| Vapor pressure |
85 h Pa (20 ° C) |
|||||||||||||||
| solubility |
soluble in water (> 30 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.392 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 5 ml m −3 or 20 mg m −3 |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Diisopropylamine is a chemical compound from the group of secondary, aliphatic amines and dialkylamines .
properties
Diisopropylamine is in the form of a volatile, caustic, highly flammable, colorless liquid with an amine-like odor. It decomposes at higher temperatures, releasing nitrogen oxides .
use
Diisopropylamine is used as a catalyst , solvent and as an intermediate in the production of corrosion inhibitors and other compounds (such as diisopropylethylamine ), as well as in amine washing and in Sonogashira coupling . In medicine , diisopropylamine is used as an arteriolar vasodilator . Diisopropylamine is used in organic chemistry as a starting compound for the production of the weakly nucleophilic strong base lithium diisopropylamide (LDA).
safety instructions
Diisopropylamine forms highly flammable vapor-air mixtures. The compound has a flash point of −7 ° C. The explosion range is between 1.2 vol.% (50 g / m 3 ) as the lower explosion limit (LEL) and 8.5 vol.% (358 g / m 3 ) as the upper explosion limit (UEL). The limit gap width was determined to be 1.02 mm (50 ° C). This results in an assignment to explosion group IIA. The ignition temperature is 285 ° C. The substance therefore falls into temperature class T3. Violent reactions can occur with oxidizing agents , acids , aluminum and organic compounds. When reacting with nitrosating reagents, carcinogenic N -nitrosamines can form.
Derivatives and related compounds
- Lithium diisopropylamide (lithium salt of diisopropylamine)
- Isopropylamine
- Propylamine
- Diisopropylethylamine (Hünig base)
- N -nitroso-diisopropylamine
Individual evidence
- ↑ a b c d e f g h Entry on diisopropylamine in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Kayelaby: Properties of organic compounds
- ↑ Entry on Diisopropylamine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 108-18-9 or diisopropylamine ), accessed on November 2, 2015.
- ↑ a b sheet diisopropylamine (PDF) at Merck , accessed on 23 February 2010 .
- ↑ Stobbe / Baumann, internal medicine, ISBN 3-86126-075-1 .
- ↑ AP Smith, JJS Lamba, CL Fraser: Efficient Synthesis of Halomethyl-2,2'-Bipyridines: 4,4'-Bis (chloromethyl) -2,2'-Bipyridine In: Organic Syntheses . 78, 2002, p. 82, doi : 10.15227 / orgsyn.078.0082 ; Coll. Vol. 10, 2004, p. 107 ( PDF ).
- ^ A b c d E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.


