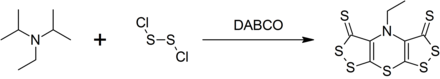Diisopropylethylamine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Diisopropylethylamine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 19 N | |||||||||||||||
| Brief description |
colorless liquid with an amine-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 129.25 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.76 g cm −3 (at 20 ° C) |
|||||||||||||||
| Melting point |
−127 ° C |
|||||||||||||||
| boiling point |
127 ° C |
|||||||||||||||
| Vapor pressure |
16 hPa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4138 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Diisopropylethylamine or Hünig's base is a tertiary amine . It is named after the German chemist Siegfried Hünig .
Due to the steric shielding, only one proton is small enough to be attacked by the nitrogen lone pair . The Hünig base is therefore used as a base in organic syntheses .
Presentation and extraction
The compound is produced by reacting diisopropylamine and acetaldehyde in the presence of hydrogen and platinum or zirconium oxide catalysts at temperatures between 100 ° C. and 150 ° C. under increased pressure.
properties
Diisopropylethylamine is a colorless liquid that boils at 127 ° C under normal pressure . According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in Torr, T in ° C with A = 7.66956, B = 1663.8374 and C = 220.897 im Temperature range from 28.57 to 149.86 K.
Diisopropylethylamine forms highly flammable vapor-air mixtures. The compound has a flash point of 9.5 ° C. The explosion range is between 0.7 vol.% As the lower explosion limit (LEL) and 6.3 vol.% As the upper explosion limit (UEL). The ignition temperature is 240 ° C. The substance therefore falls into temperature class T3.
Reactions
Due to the very low nucleophilicity of diisopropylethylamine, it is often used in alkylation reactions of secondary amines with alkyl halides , in which a hydrohalic acid such as hydrochloric acid (HCl), hydrobromic acid (HBr) or hydroiodic acid (HI) is formed. This is bound by the basic nitrogen function and the corresponding ammonium salts are formed. Without the addition of diisopropylethylamine, part of the secondary amine used as starting material would take over this role by binding the hydrogen halide formed. Since it is then present in protonated form, it cannot react further to form the tertiary amine, which lowers the yield.
It is not only used as a base, but also as a synthetic building block in the one-pot reaction to produce the heterocyclic scorpionin . To do this, it is reacted with dichlorodisulfide (Cl – S – S – Cl) in the presence of the DABCO catalyst .
literature
- Jason L. Moore, Stephen M. Taylor, Vadim A. Soloshonok: An efficient and operationally convenient general synthesis of tertiary amines by direct alkylation of secondary amines with alkyl halides in the presence of Huenig's base. In: Arkivoc. 2005, 2005, p. 287, doi : 10.3998 / ark.5550190.0006.624 .
Individual evidence
- ↑ a b Data sheet N-Ethyldiisopropylamine (PDF) from Merck , accessed on March 22, 2010.
- ↑ a b c d e f g h i Entry on ethyldiisopropylamine in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b Entry on Hünig Base. In: Römpp Online . Georg Thieme Verlag, accessed on October 24, 2016.
- ↑ Kirk L. Sorgi: Encyclopedia of Reagents for Organic Synthesis . John Wiley & Sons, Ltd, 2001, ISBN 978-0-470-84289-8 , Diisopropylethylamine.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-246.
- ↑ patent WO2006 / 136571 A1, BASF SE of 2006.
- ↑ patent US2010 / 267948 A1, BASF SE of 2010.
- ^ Yaws, CL: The Yaws Handbook of Vapor Pressure - Antoine Coefficients Elsevier 2015, p. 76, ISBN 978-0-12-802999-2 .
- ↑ W. Rees, Carlos F. Marcos, Cecilia Polo, Tomás Torroba, Oleg A. Rakitin: From Hünig's Base to Bis ([1,2] dithiolo) - [1,4] thiazines in One Pot: The Fast Route to Highly Sulfurated heterocycles . In: Angewandte Chemie International Edition in English , 1997 , 36 (3) , 281–283. doi : 10.1002 / anie.199702811 .