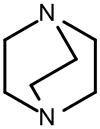
1,4-diazabicyclo (2.2.2) octane
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 1,4-diazabicyclo [2.2.2] octane ( IUPAC ) | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 12 N 2 | ||||||||||||||||||
| Brief description |
white solid with an ammonia-like odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 112.17 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.14 g cm −3 |
||||||||||||||||||
| Melting point |
159.8 ° C |
||||||||||||||||||
| boiling point |
174-176 ° C |
||||||||||||||||||
| Vapor pressure |
0.68 h Pa (21 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
1,4-Diazabicyclo [2.2.2] octane , also known as triethylenediamine ( TEDA ), is a bicyclic, tertiary amine . The abbreviation “ DABCO ”, which is frequently used in technology, is derived directly from the official IUPAC name and is a registered brand name .
Physical Properties
TEDA is a solid white at room temperature that melts at 159.8 ° C. It is easily soluble in water . Due to the spatial structure of the molecule, the lone pairs of electrons in nitrogen are sterically unhindered, which is reflected in a pronounced nucleophilicity .
Manufacturing
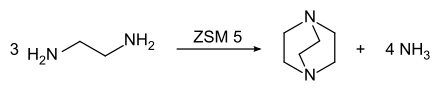
TEDA is produced industrially by converting ethylenediamine on ZSM 5 zeolite catalysts :
The compound can also be obtained by heating N -hydroxyethylpiperazine .
use
TEDA is used as a catalyst for the production of polyurethane plastics and as a labile ligand or as a base, for example in the Baylis-Hillman reaction . In organic synthesis it is used as a reagent for the cleavage of esters , the decarboxylation of geminal diesters, for the production of azirines from vinyl azides and numerous other reactions.
Brand name
The abbreviation “DABCO”, which is frequently used in technology, is derived directly from the official IUPAC name 1,4- D i a za b i c yclo [2.2.2] o ctan and is a registered brand name of the company Evonik Industries AG for amine-based Catalysts.
Individual evidence
- ↑ a b c d e f g h Entry on 1,4-diazabicyclo (2.2.2) octane in the GESTIS substance database of the IFA , accessed on February 3, 2017(JavaScript required) .
- ↑ Data sheet 1,4-diazabicyclo (2.2.2) octane (PDF) from Merck , accessed on June 15, 2017.
- ↑ a b c d Entry on 1,4-diazabicyclo [2.2.2] octane. In: Römpp Online . Georg Thieme Verlag, accessed December 7, 2017.
- ↑ Patent EP0423526 : A process for preparing triethylenediamine and piperazine. Registered on September 29, 1990 , published on November 18, 1993 , applicant: Bayer AG, inventors: Jens Weitkamp , Stefan Ernst, Dieter Lindner, Hans-Josef Buysch, Artur Botta, Lotharuppe.
- ↑ Innovative polyurethane foam additives. Retrieved January 11, 2018 .