Baylis-Hillman reaction
The Baylis-Hillman reaction , also Morita-Baylis-Hillman reaction , is a reaction from the field of organic chemistry and was named after its discoverers Anthony B. Baylis and Melville ED Hillman . It is used for the synthesis of α- hydroxy vinyl carbonyls . This requires a vinyl ketone or ester , an aldehyde or ketone and a base . Triethylenediamine (DABCO) is traditionally used as the base , recently also DBU and DMAP .
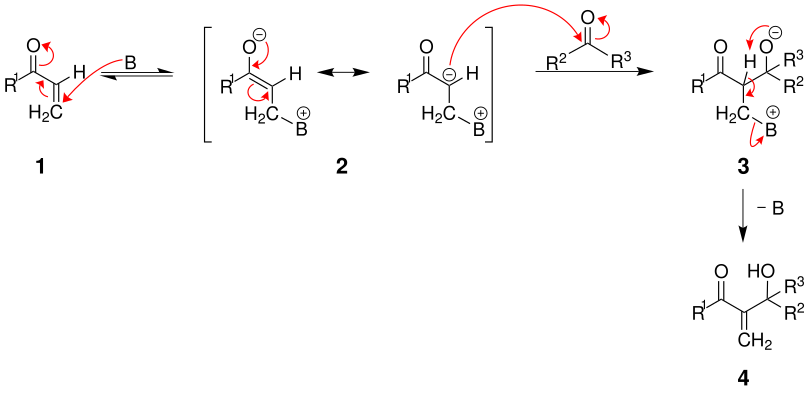
mechanism
The following illustration shows a plausible course of the reaction:
In the first step, the base (in this case DABCO, abbreviated as B) attacks the vinyl ketone 1 nucleophilically , so that the mesomeric-stabilized enolate 2 is formed. This now attacks the aldehyde / ketone with formation of the alcoholate 3 nucleophilic. During the proton transfer, the base is split off and the product 4 is formed. The reaction is very slow, but can be accelerated with ultrasound.
The reaction also takes place with imines or imine derivatives instead of aldehydes / ketones.
literature
- Patent DE2155113A1 : Process for the production of acrylic compounds. Published May 10, 1972 , Inventor: AB Baylis, MED Hillman.
Web links
- organic-chemie.ch: Baylis-Hillman reaction
Individual evidence
- ↑ a b L. Kürti , B. Czakó: Stratigic Applications of Named Reactions in Organic Synthesis . Elsevier Academic Press, Amsterdam 2005, ISBN 978-0-12-429785-2 , pp. 48-49.
- ↑ T. Laue, A. Plagens: Name and keyword reactions . 5th edition, BG Teubner Verlag / GWV Fachverlage, Wiesbaden 2006, ISBN 978-3-8351-0091-6 , pp. 34-35.
- ↑ Fernando Coelho, Wanda P. Almeida, Demetrius Veronese, Cristiano R. Mateus, Elizandra C. Silva Lopes, Rodrigo C. Rossi, Gabriel PC Silveira, César H. Pavam: Ultrasound in Baylis – Hillman reactions with aliphatic and aromatic aldehydes: scope and limitations. In: Tetrahedron. 58, No. 37, 2002, pp. 7437-7447, doi : 10.1016 / S0040-4020 (02) 00822-0 .