2-methylfuran
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
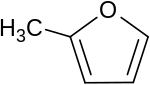
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-methylfuran | |||||||||||||||
| other names |
Silvan |
|||||||||||||||
| Molecular formula | C 5 H 6 O | |||||||||||||||
| Brief description |
colorless liquid with an ethereal odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 82.10 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.91 g cm −3 |
|||||||||||||||
| Melting point |
−89 ° C |
|||||||||||||||
| boiling point |
64 ° C |
|||||||||||||||
| Vapor pressure |
139 mbar (20 ° C) |
|||||||||||||||
| solubility |
sparingly soluble in water (3 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.4332 (20 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2-Methylfuran is a chemical compound belonging to the furans group and is isomeric to 3-Methylfuran .
Occurrence
2-methylfuran occurs naturally in the form of an odor component in cigarette smoke and mold.
Extraction and presentation
2-Methylfuran can be obtained by a palladium- catalyzed reaction of penta-1,3-diene with hydrogen peroxide .
Industrially, 2-methylfuran is produced from furfuryl alcohol by catalytic hydrogenation with a copper catalyst in the vapor phase .
properties
2-Methylfuran is a volatile, colorless liquid with an ethereal odor that is sparingly soluble in water. It decomposes when heated strongly and has a dynamic viscosity of 4 mPa · s at 20 ° C.
use
2-Methylfuran was used as a high octane gasoline additive during World War II . It is also used to produce 2-methylthiophene .
safety instructions
The vapors of 2-methylfuran form an explosive mixture with air (flash point −22 ° C).
Individual evidence
- ↑ a b c d e f g h i j k Entry on 2-methylfuran in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ a b Data sheet 2-methylfuran (PDF) from Merck , accessed on October 12, 2012.
- ↑ Wolfgang Legrum: Fragrances, between stench and fragrance . Springer DE, 2011, ISBN 3-8348-1245-5 , p. 67 ( limited preview in Google Book search).
- ^ CW Jones: Applications of Hydrogen Peroxide and Derivatives . Royal Society of Chemistry, 1999, ISBN 0-85404-536-8 , pp. 102 ( limited preview in Google Book search).
- ↑ Patent US6852868 : Catalytic hydrogenation of furfural to produce 2-methylfuran and 2-methyltetrahydrofuran, using a reduced copper based catalyst. Filed August 21, 2002 , published February 8, 2005 , applicant: Pure Energy Corporation, inventor: Irshad Ahmed.
- ↑ Peter Kurzweil, Paul Scheipers: Chemistry: Fundamentals, structural knowledge, applications and experiments . Springer DE, 2012, ISBN 3-8348-1555-1 , p. 274 ( limited preview in Google Book search).
- ^ KB Wiberg, HF McShane: 2-Chloromethylthiophene In: Organic Syntheses . 29, 1949, p. 31, doi : 10.15227 / orgsyn.029.0031 ; Coll. Vol. 3, 1955, p. 197 ( PDF ).

