Acrylic acid methyl ester
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Acrylic acid methyl ester | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 6 O 2 | |||||||||||||||
| Brief description |
colorless, pungent smelling, volatile, highly flammable liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 86.09 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.95 g cm −3 |
|||||||||||||||
| Melting point |
−75 ° C |
|||||||||||||||
| boiling point |
80 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
moderate in water (52 g l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.3984 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−362.2 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Acrylic acid methyl ester , often also called methyl acrylate , is a chemical compound from the group of acrylic acid esters and thus also the carboxylic acid esters . It is in the form of a colorless, pungent smelling liquid .
Manufacturing
Acrylic acid methyl ester can be produced by debromination of 2,3-dibromopropanoic acid methyl ester with zinc and sulfuric acid.
The pyrolysis of lactic acid methyl ester in the presence of ethenone ( ketene ) produces methyl acrylate in good yield. Milchsäuremethylester place in recent attention as a chemical compound derived from renewable raw materials ( English "green chemical" ). A more recent patent describes the pyrolysis of methyl lactate on zeolites , which produces methyl acrylate in 93% yield.
The nickel tetracarbonyl- catalyzed hydrocarboxylation of acetylene with carbon monoxide in the presence of methanol also yields methyl acrylate, which Walter Reppe found . The patent literature describes a single-stage synthesis route to methyl acrylate via the oxidation of propene or acrolein with oxygen in the vapor phase in the presence of methanol. The reaction of methyl formate with acetylene in the presence of transition metal catalysts also leads to methyl acrylate. The alcoholysis of propiolactone with methanol, like the methanolysis of acrylonitrile via the intermediate acrylamide sulfate, is an outdated process for the production of methyl acrylate.
The availability of inexpensive acrylic acid using the propene oxidation process has made direct esterification with methanol under acidic catalysis ( sulfuric acid , p-toluenesulfonic acid , acidic ion exchangers ) the standard reaction that is exclusively used on an industrial scale.
properties
Acrylic acid methyl ester is a colorless, pungent odor, volatile, highly flammable liquid that boils at 80 ° C at normal pressure . According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 4.32277, B = 1338.663 and C = −43.516 in the temperature range from 229.5 to 353.3 K. The vapors are three times as heavy as air. Acrylic acid methyl ester tends to polymerize spontaneously, especially under the influence of light or at elevated temperatures. The heat of polymerization is −80 kJ · mol −1 or –930 kJ · kg −1 . Therefore 10-20 ppm hydroquinone monomethyl ether ( MEHQ ) is added as a stabilizer . When stored in the presence of oxygen and below 35 ° C, the storage period is at least one year.
Safety-related parameters
Acrylic acid methyl ester forms highly flammable vapor-air mixtures. The compound has a flash point of −3 ° C. The explosion range is between 1.95% by volume (71 g / m 3 ) as the lower explosion limit (LEL) and 16.3% by volume (581 g / m 3 ) as the upper explosion limit (UEL). A correlation with the vapor pressure function results in a lower explosion point of −5 ° C). The limit gap width was determined to be 0.85 mm. This results in an assignment to explosion group IIB. The ignition temperature is 415 ° C. The substance therefore falls into temperature class T2.
use
After butyl acrylate and ethyl acrylate, acrylic acid methyl ester is the third most important acrylic ester with a worldwide annual production of approx. 200,000 tons per year.
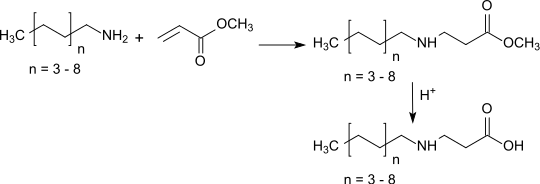
Like ethyl acrylate, the methyl ester also reacts under catalysis by Lewis bases in a Michael addition with amines in high yields to form β-alanine derivatives, which when using long-chain amines and subsequent hydrolysis of the ester function produce amphoteric surfactants .
Acrylic acid methyl ester is used in considerable quantities over 50,000 tons / year for the production of 2- dimethylaminoethyl acrylate by transesterification with dimethylaminoethanol .
Its range of use as a comonomer in the polymerization with a large number of acrylic and vinyl monomers is largely the same as that of ethyl acrylate . With methyl acrylate as a comonomer, harder and more brittle acrylic paints are obtained than with the homologous acrylic acid esters . Copolymerization of methyl acrylate with acrylonitrile improves the processability in the melt into fibers that could be of interest as precursors for carbon fibers (“carbon fibers”).
Methyl acrylate is used to produce polyamidoamine - dendrimers (typically by a Michael addition with a primary amine) were used.
safety instructions
The substance is easily degraded in the environment and does not accumulate. Due to its irritating properties to the eyes, skin and mucous membranes and the unpleasant pungent odor, methyl acrylate must be handled in closed compartments.
Web links
- Entry on methyl acrylate in the Spectral Database for Organic Compounds (SDBS) of the National Institute of Advanced Industrial Science and Technology (AIST)
- Said Abbadi: Production and use of polymer peroxides as initiators of the radical polymerization of methyl methacrylate systems . (PDF, 1.3 MB) Dissertation, Hamburg, 2003. urn : nbn: de: gbv: 18-21304
See also
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q r s t Entry on methyl acrylate in the GESTIS substance database of the IFA , accessed on August 01, 2017(JavaScript required) .
- ↑ CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ Entry on methyl acrylate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 96-33-3 or methyl acrylate ), accessed on September 16, 2019.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-25.
- ^ F. Beilstein: Handbook of organic chemistry , 3rd edition, 1st volume. Verlag Leopold Voss, 1893, p. 501. Full text .
- ↑ Patent US2417748 : Preparation of Methyl Acrylate. Published March 18, 1947 , Applicant: Eastman Kodak Company, Inventor: Hugh J. Hagemeyer.
- ↑ Patent US5250729 : Process for preparing unsaturated carboxylic acid or ester thereof. Filed December 4, 1989 , published October 5, 1993 , Applicant: Mitsubishi Gas Chemical Company , Inventor: Takafumi Abe, Shinichi Hieda.
- ^ W. Reppe, Liebigs Ann. Chem. , 582 (1), 116-132 (1953).
- ↑ Patent US3925463 : Process for the Production of Methyl Acrylate. Published on December 9, 1975 , Applicant: Societá Italiana Resine SIR, SpA, Inventors: Natale Ferlazzo, Buzzi Gian Fausto, Ghirga Marcello.
- ↑ Patent US6022990 : Method for synthesizing methyl acrylate. Published on February 8, 2000 , Applicant: Chengdu Institute of Organic Chemistry, Inventor: Zhao-Tie Liu, Jia-Qi Zhang, Xian-Gui Yang.
- ↑ H.-J. Arpe, Industrial Organic Chemistry , 6th edition, Wiley-VCH Verlag, Weinheim, 2007, ISBN 978-3-527-31540-6 .
- ↑ Esterification: Acrylate esters (MA, EA, BA, MMA, 2-EHA) ( English ) amberlyst.com. Retrieved February 21, 2013.
- ↑ Stull, DR: Vapor Pressure of Pure Substances - Organic Compounds in Ind. Eng. Chem. 39 (1947) 517-540, doi : 10.1021 / ie50448a022 .
- ↑ Trade Association Raw Materials and Chemical Industry , Leaflet R 008 Polyreactions and Polymerizable Systems , Edition 05/2015, ISBN 978-3-86825-069-5
- ^ BASF AG, Technical Data Sheet, Methyl acrylate , August 2003.
- ^ A b c d E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ CEH Marketing Research Report Acrylic Acid and Esters, SRI Consulting July of 2007.
- ↑ Patent WO2010136696 : Composition Including Dialkyl Tin Oxide and Use Thereof as a Transesterification Catalyst for the Synthesis of (meth) acrylic Esters. Published on December 2, 2010 , applicant: Arkema France, inventor: Jean-Michel Paul, Boris Tonnelier, Francis Augustin.
- ↑ DOW Methyl acrylate, Product Safety Assessment.
- ↑ VA Bhanu et al .: Synthesis and characterization of acrylonitrile methyl acrylate statistical copolymers as melt processable carbon fiber precursors . In: polymer . tape 43 , no. 18 , August 2002, p. 4841-4850 , doi : 10.1016 / S0032-3861 (02) 00330-0 (English, PDF ).