Ethenone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ethenone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 2 O | |||||||||||||||
| Brief description |
extremely flammable, colorless gas with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 42.04 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
1.93 kg m −3 |
|||||||||||||||
| Melting point |
−151 ° C |
|||||||||||||||
| boiling point |
−56 ° C |
|||||||||||||||
| solubility |
hydrolyzed in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 0.5 ml m −3 or 0.9 mg m −3 |
|||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−47.5 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
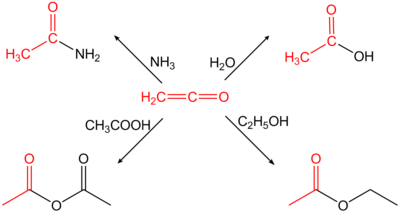
Ethenone or ketene (emphasis on the second syllable: ket e n ) is the simplest chemical compound from the group of ketenes and can be understood as the internal anhydride of acetic acid .
Extraction and presentation
Ketene is produced by the dehydration of acetic acid or the pyrolysis of acetone . For example, when acetone vapors are passed through heated pipes or electrically heated metal wires at 500-600 ° C. in the presence of a little carbon disulfide, ethenone and methane are produced in yields of up to 95% of theory. educated. In industrial chemistry, ketone pyrolysis has largely been replaced by the dehydration of acetic acid (Schmidlin-Bergman-Wilsmore reaction).
Ethenone was found around the same time by Hermann Staudinger - through the reaction of bromoacetyl bromide with metallic zinc - and Norman Thomas Mortimer Wilsmore - through the thermal decomposition of acetic anhydride .
properties
Ethenone is chemically unstable. The gas is only reasonably stable at low temperatures (−80 ° C). It must therefore always be newly produced for each use and further processed immediately, since otherwise dimerization to diketene or a reaction to difficult-to-handle polymers occurs. The polymer content in the production of diketene is therefore also z. B. reduced by adding sulfur dioxide to the ketene gas or the reaction medium. Because of its accumulated double bonds, ethenone is very reactive and adds H-acidic compounds nucleophilically to the corresponding acetic acid derivatives; so it hydrolyzes e.g. B. with water to acetic acid or reacts with prim. or sec. Amines to the corresponding acetamides.
Ketene is highly toxic; the toxicity is about eight times that of phosgene .
use
Ethenone is used to produce acetic anhydride from acetic acid . Generally it is used for the acetylation of chemical compounds.
Ethenone reacts with formaldehyde in the presence of Lewis acids such as AlCl 3 , ZnCl 2 or BF 3 as catalysts to form β- propiolactone . The technically most important use of ethenone is the synthesis of sorbic acid through the reaction of crotonaldehyde (2-butenal) with ketene in toluene at approx. 50 ° C in the presence of zinc salts of long-chain carboxylic acids. This creates a polyester of 3-hydroxy-4-hexenoic acid, which is thermally or hydrolytically depolymerized to sorbic acid.
safety instructions
Ethenone tends to polymerize spontaneously . Contact with hydrogen peroxide leads to an explosive reaction. The vapors of ketene can form an explosive mixture with air.
Individual evidence
- ↑ a b c d e Entry on ketene in the GESTIS substance database of the IFA , accessed on February 28, 2017(JavaScript required) .
- ↑ There is not yet a harmonized classification for this substance . A labeling of Ketene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), which was accessed on February 27, 2018, is reproduced from a self-classification by the distributor .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 463-51-4 or ethenone ), accessed on November 2, 2015.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-21.
- ↑ K.-H. Lautenschläger, W. Schröter, A. Wanninger, "Taschenbuch der Chemie", 20th edition 2006, ISBN 978-3-8171-1761-1 .
- ↑ CD Hurd: Ketene In: Organic Syntheses . 4, 1925, p. 39, doi : 10.15227 / orgsyn.004.0039 ; Coll. Vol. 1, 1941, p. 330 ( PDF ).
- ↑ J. Schmidlin, M. Bergman, Ber. German Chem. Ges. , 43 , 2821 (1910), DOI: 10.1002 / cber.19100430340 .
- ^ Tidwell, TT (2005), A Century of Ketene (1905-2005): The Discovery of a Versatile Class of Reactive Intermediates. Angewandte Chemie, 117: 5926-5933. doi: 10.1002 / anie.200500098 and H. Staudinger, HW Klever, Ber. German Chem. Ges., 41 , 5943 (1908).
- ↑ NTM Wilsmore, J. Chem. Soc. , 91 , 1938 (1907), DOI: 10.1039 / ct9079101938 .
- ↑ Patent EP0377438 : Published on June 11, 1990 , Applicant: Lonza AG, inventor R. Bergamin et al ..
- ↑ a b Entry on Diketen. In: Römpp Online . Georg Thieme Verlag, accessed on June 16, 2014.
- ↑ Hans-Jürgen Arpe, "Industrial Organic Chemistry", 6th edition, 2007, WILEY-VCH Verlag, Weinheim, ISBN 978-3-527-31540-6 .
- ↑ Patent EP1295860 : Published on March 26 , Applicant: Nutrinova GmbH, inventors D. Decker et al ..