Sorbic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
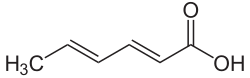
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Sorbic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 8 O 2 | |||||||||||||||
| Brief description |
white solid with a slightly sour, scratchy taste |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 112.13 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.2 g cm −3 (at 20 ° C) |
|||||||||||||||
| Melting point |
134 ° C |
|||||||||||||||
| boiling point |
228 ° C (decomposition) |
|||||||||||||||
| Vapor pressure |
1 Pa (20 ° C) |
|||||||||||||||
| pK s value |
4.76 (20 ° C) |
|||||||||||||||
| solubility | ||||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
The sorbic acid (also hexadienic ) is a di-unsaturated carboxylic acid . It is used both as a free acid under the name E 200, which is valid in the EU, and in the form of its salts - the sorbates - as a preservative or food additive .
Properties of the acid
Sorbic acid is poorly soluble in cold water, but easily soluble in hot water. Sorbic acid is also readily soluble in alcohols, concentrated acetic acid ( glacial acetic acid ), acetone and toluene . It absorbs light at a maximum of 264 nm, the pKa value is 4.76. The acid has a slightly sour, "scratchy" taste, which, however, is imperceptible at the concentrations used for the preservation of food. The flash point is 127 ° C, the ignition temperature 415 ° C.
synthesis
In the laboratory, sorbic acid is represented by the Doebner variant of Knoevenagel condensation . To do this, malonic acid is dissolved in pyridine and then reacted with crotonaldehyde and piperidine (as a catalyst ). Another possibility is the reaction of ketene and 2-butenal in an inert solvent and a catalyst (for example a zinc (II) salt). This gives a polymeric ester of 3-hydroxy-4-hexenoic acid which can be converted into sorbic acid by heating or alkali treatment.
Natural occurrence
A precursor to sorbic acid, parasorbic acid , is naturally found in rowan berries . The rowanberry Sorbus aucuparia was also the namesake of sorbic acid. The lactone is correctly called 5,6-dihydro-6-methyl-2 H -pyran-2-one and is an oily liquid with a sweet, aromatic odor. From this compound , formerly known as sorbin oil , free sorbic acid was prepared for the first time in 1859. Finally, sorbic acid is found in wine and chemically bound in the fat of some species of aphids (aphids).
Use as a preservative
Sorbic acid is mainly used as a preservative for food and feed, pharmaceuticals, cosmetics and detergents. Sorbic acid itself ( E 200 ) or one of its more water-soluble salts is used. Potassium sorbate (E 202) and calcium sorbate (E 203) are of industrial importance . Sodium sorbate (E 201) is sensitive to oxidation and is not produced industrially. In contrast to benzoic acid, sorbic acid can not be smelled or tasted by many people.
With sorbic acid z. B. Baked goods, margarine, cheese and sausage products are preserved. The harmlessness of sorbic acid to health has been proven in various feeding studies. The allergenic potential is classified as low, as it is used in the human body like a fatty acid derived from food. In rare cases, however, it can cause allergies and, as an acid, irritate the mucous membranes or skin of very sensitive people. The permitted daily dose of sorbic acid is 25 mg / kg body weight. Sorbic acid is approved as a food additive in almost all European countries and has so-called GRAS status in the USA .
Another important area of application is the stabilization of wine against secondary fermentation caused by yeasts that are still present. In the production of wine, the addition of a maximum of 0.2 g / l (Germany and Austria) or 1 g / l (USA) to the must or wine is therefore permitted. However, since the acid does not work against lactic acid bacteria , undesirable and irreversible changes in taste ( geranium tone ) of the wine can occur, which is why the wine is usually additionally stabilized with sulfur dioxide .
The Tobacco Ordinance allows the use of sorbic acid as a preservative for tobacco products.
Effect of sorbic acid against microorganisms
Sorbic acid is an antimicrobial substance ; their effect is based on various factors. On the one hand, it is directed against various enzymes in the cells of microorganisms . It mainly affects the carbohydrate metabolism enzymes, such as the enzyme emulase . In addition, sorbic acid intervenes in the citric acid cycle . There it inhibits, among other things, the enzyme isocitrate dehydrogenase , and thus the step from isocitric acid to oxalosuccinic acid , as well as the enzyme α-ketoglutarate dehydrogenase , i.e. the conversion of α-ketoglutaric acid to succinic acid .
Furthermore, with its double bonds , sorbic acid enters into covalent bonds with SH groups of enzymes, which in turn inactivate them. It is assumed that the inhibiting effect of sorbic acid against the microorganisms is due to the inhibition of several enzymes. It is also assumed that sorbic acid has an effect on the cell wall . In fact, it inhibits the uptake of amino acids such as serine or alanine even at very low concentrations .
In order for it to be effective in the microorganism cell at all, it must penetrate the cell wall . Preferably the undissociated acid moiety enters the cell. This is also the most important when it comes to the effectiveness of the substance. At a pH of 3.15, 40% of the sorbic acid present can get into the cell interior. This shows the dependency of the preservation on the pH value.
If sorbic acid is present in low concentrations and at the same time high bacterial counts, the microorganisms can include sorbic acids in their metabolism. This means that sorbic acid is only suitable for maintaining hygienically perfect food and not for restoring sterile conditions, as is in any case inadmissible in the practice of food preservation .
literature
- Chemical food preservation , Lück, Jager, Springer-Verlag Heidelberg, 3rd edition 1995, ISBN 3-540-57607-X , pages: 158-174.
- Sorbate Food Preservatives , John N. Sofos, CRC Press, 1988, ISBN 0-8493-6786-7 .
Individual evidence
- ↑ Entry on E 200: Sorbic acid in the European database for food additives, accessed on June 27, 2020.
- ↑ a b c d e f g h i Entry on sorbic acid in the GESTIS substance database of the IFA , accessed on December 16, 2019(JavaScript required) .
- ↑ a b c d e f F. v. Bruchhausen, G. Dannhardt, S. Ebel, AW Frahm, E. Hackenthal, U. Holzgrabe: Hagers Handbook of Pharmaceutical Practice. Vol. 9: Substances P-Z. 5th edition, Birkhäuser / Springer, 1991, ISBN 3-540-52688-9 , p. 634.
- ↑ Data sheet sorbic acid (PDF) from Merck , accessed on January 19, 2011.
- ↑ Zusatzstoffe-Online.de: Sorbic acid .

