Succinic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
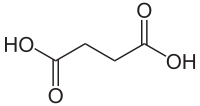
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Succinic acid | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 4 H 6 O 4 | |||||||||||||||||||||
| Brief description |
colorless and odorless crystalline solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 118.09 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.56 g cm −3 |
|||||||||||||||||||||
| Melting point |
185-190 ° C |
|||||||||||||||||||||
| boiling point |
235 ° C |
|||||||||||||||||||||
| pK s value |
|
|||||||||||||||||||||
| solubility |
soluble in water (58 g l −1 at 20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
−940.5 kJ / mol |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Succinic acid , also known as succinylic acid or butanedioic acid , E 363 , is a colorless, crystalline aliphatic dicarboxylic acid . The crystals are readily soluble in boiling water.
history
Succinic acid was discovered in 1546 by Georgius Agricola during dry distillation by heating amber . Nicolas Lémery was the first to recognize the acidic nature of the substance in 1675 and Jöns Jakob Berzelius found out the composition (C 4 H 6 O 4 ) of the acid. Medicinal products based on succinic acid and its salts for use in catarrh and syphilis were in use well into the 20th century .
At the end of the 19th century, the pharmacist Otto Helm, who lives in Gdansk, tried to identify the origin of amber based on its content of succinic acid. The acid obtained by dry distillation and sublimed in retorts weighed Helm and came to the conclusion that a proportion between 3.2% and 8.2% suggests Baltic amber . Using this method, he also examined Sicilian amber and archaeological finds from Mycenae, among others . While he found no traces of acid in samples of Sicilian amber, the ancient pieces had an acid content of 4.1% to 6.3%. Helm believed that the answer to the question of the origin of the amber finds made in the Mediterranean, which has been an issue for archaeologists since ancient times. According to these findings, it must have been Baltic amber. It later turned out that the content of succinic acid is not a reliable feature for identifying Baltic amber, since corresponding concentrations of succinic acid were also found in amber from other European deposits. Helm's proof of origin remained valid, since these deposits were not yet known in ancient times.
Rolf CA Rottländer came to the conclusion in 1970 that succinic acid is not actually contained in amber by nature, but only forms during the alkaline hydrolysis of amber (as an alkaline salt) or during dry distillation (as its anhydride). He suggested that succinic acid (as a salt or anhydride) should be viewed as a natural oxidation product of amber and thus as an indicator of its aging process.
The question of the origin of ancient amber from archaeological excavations is therefore still the subject of scientific discussions and has recently been tried to answer with the help of other investigation methods ( infrared spectroscopy , mass spectrometry , gas chromatography , nuclear magnetic resonance spectroscopy [NMR] and others).
Occurrence
The name of succinic acid is derived from amber , a gemstone made from fossil resin that contains succinic acid. Traditionally, different types of amber were distinguished on the basis of chemical substances depending on the content of succinic acid in succinites (3% to 8%) and retinites (fossil resins with a succinic acid content of less than 3% or without succinic acid). Succinic acid is also found in many brown coals .
In the metabolism of all organisms, the salt of succinic acid occurs during the breakdown of glucose as an intermediate stage in the regeneration of the acceptor oxaloacetic acid . It is accordingly an intermediate metabolic product of the citrate cycle , and it also occurs in the urea cycle . In nature, succinic acid is also found in many plant juices ( rhubarb , tomatoes) as well as in algae and mushrooms.
Succinic acid can also be an end product of the metabolism of some anaerobically living bacteria, for example in beef rumen . This represents a system in which a number of facultatively anaerobic bacteria obtain their nutritional basis from the food pulp. The β-glycosidic bonds of the cellulose in the food are broken by the rumen flora , especially the fungi it contains . The resulting grape sugar (glucose) serves as a substrate for microorganisms. The products of bacterial metabolism are mainly short-chain carboxylic acids such as acetic acid and succinic acid as well as ethanol . The succinic acid produced by bacteria in turn serves as an energy source for other bacteria, which convert it into propionic acid.
Extraction and presentation
Technically
Various synthetic routes for the production of succinic acid are known technically . The production is usually carried out via a catalytic hydrogenation of maleic acid , maleic anhydride or fumaric acid , whereby various catalysts can be used (Ni, Cu, NiO, CuZnCr, Pd-Al 2 O 3 , Pd-CaCO 3 ). This synthetic route produces 20,000 to 30,000 tons of succinic acid per year.
In addition, the oxidation of 1,4-butanediol (BDO) is possible, whereby various technical routes exist. The hydrocarboxylation of acetylene glycol , catalyzed by RhCl 3 - pentachlorothiophenol , acetylene , acrylic acid , 1,4-dioxane and propiolactone is also possible.
Biotechnological
Succinic acid can be made from carbohydrates through fermentation , especially starch and various oligosaccharides (C6 and C5 sugars). The natural occurrence of succinic acid in the metabolism can be used here in order to have it specifically produced by microorganisms. Biotechnologically produced succinic acid is mainly used for use in foodstuffs, but there are now also pilot plants for biotechnological succinic acid production for technical applications.
Due to the possibility of biotechnologically producing succinic acid on a large scale with the help of bacteria, research is being intensified with Basfia succiniciproducens , Mannheimia succiniciproducens and Anaerobiospirillum succiniciproducens . The research also focuses on the model organism Escherichia coli , which is to be optimized for the production of high quantities of succinic acid via metabolic engineering .
Chemical properties
The salts and esters of succinic acid are called succinates . The term 'succinate' is derived from the Latin word suc (c) inum for amber . In their crystal lattices they contain the succinate ion as a negatively charged anion . The general formula of an alkali succinate is MOOC – CH 2 –CH 2 –COOM, M especially stands for sodium and potassium ions. Alkali succinates dissolve easily in water. The alkaline earth succinates are difficult, the other succinates are not at all water soluble. Calcium succinate can be found in unripe fruits or algae . The ester-like succinates can be described by the semi-structural formula R-O-CO-CH 2 -CH 2 -CO-O-R.
If succinic acid is heated, it splits off water and forms succinic anhydride with ring closure .
use
Use in the food sector
Succinic acid is approved in the EU as a food additive with the number E 363 and, due to its mildly acidic and at the same time slightly salty taste, serves as a flavor enhancer for desserts, dry soups and powdered drinks . Various salts of succinic acid are used as table salt substitutes in diet foods ( Fe , Mg , Ca , K ). Due to the body's own production and metabolism of succinic acid, the use of succinic acid as a food additive is considered safe.
During alcoholic fermentation and when the wine is later matured in containers such as wooden barrels or stainless steel containers, the central acids in wine ( tartaric acid , malic acid and citric acid ), succinic acid , acetic acid , butyric acid and lactic acid are also produced . Succinic acid is mainly formed during the carbonic acid maceration and tastes slightly bitter and salty, the esterification to succinic acid monomethyl ester brings a mild, fruity component to the wine.
Technical uses
Succinic acid is a platform chemical with an annual demand of currently around 15,000 tons and a market value of six to nine euros per kilogram. B. used for the production of polyester and alkyd resins . Some succinates esterified with polyalcohols are used as solvents and plasticizers for plastics and waxes , other esters are used in perfume production . In the form of sulfosuccinic acid esters , succinic acid is also used as an important group of surfactants , but these are usually made on the basis of maleic acid.
Succinic acid is also one of the main hopes for industrial biotechnology as a platform chemical and thus as a raw material for various industrially produced chemicals and polymers . It can be used as a raw material for biotechnological production. of 1,4-butanediol (BDO), 1,4-butanediamine , tetrahydrofuran (THF), N -methyl-2-pyrrolidone (NMP), γ-butyrolactam , γ-butyrolactone (GBL) and some other products. As a basis for various products in the chemical and pharmaceutical industries as well as for bio-based plastics such as polyamides (PA), polyesters and co-polyesters as well as polyesteramides , succinic acid is interesting as a biotechnologically manufactured product and a market potential of several hundred thousand tons is forecast.
Together with other representatives of the C4-dicarboxylic acids such as fumaric and malic acid , succinic acid was identified by the US Department of Energy in 2004 as one of twelve platform chemicals with particular biotechnological manufacturing potential. In a revision of the list from 2010, succinic acid is also one of the ten products in biorefinery technology with the highest potential.
Individual evidence
- ↑ Entry on SUCCINIC ACID in the CosIng database of the EU Commission, accessed on March 21, 2020.
- ↑ Entry on E 363: Succinic acid in the European database for food additives, accessed on August 6, 2020.
- ↑ a b c d e f g Entry on succinic acid in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ a b CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-26.
- ↑ K. Andrée: The amber and its meaning in the natural sciences and humanities, art and applied arts, industry and trade. Koenigsberg 1937.
- ↑ Otto Helm: Mittheilungen über Bernstein. XII. About the origin of the amber found in the old royal graves of Mykenae and about the succinic acid content of various fossil resins , in: Writings of the Natural Research Society in Danzig , Volume VI, Issue 2, Danzig 1884, pp. 234-239.
- ↑ Curt W. Beck: To determine the origin of amber. In: Bernstein - Tränen der Götter , Bochum 1996, pp. 59–61.
- ↑ George O. Poinar: Life in Amber. Berkeley (USA) 1992.
- ^ RCA Rottländer: "On the formation of amber from Pinus resin", in: Archaeometry , 12 , pp. 35-51; doi: 10.1111 / j.1475-4754.1970.tb00004.x ; (quoted in Poinar 1992).
- ↑ a b c Boy Cornils, Peter Lappe: Dicarboxylic Acids, Aliphatics. In: Ullmann's Encyclopedia of Industrial Chemistry , Wiley-VCH, Weinheim 2005, doi : 10.1002 / 14356007.a08_523.pub2 .
- ^ A b Article succinic acid in Brockhaus Enzyklopädie online, Bibliographisches Institut & FA Brockhaus AG 2005–2009.
- ↑ a b Ana Cukolovic, Christian V. Stevens: Feasibility of production methods for succinic acid: a marriage of renewable resources and chemical technology , in: Biofuels, Bioproducts and Biorefining 2 (6), 2008; Pp. 505-529; doi: 10.1002 / bbb.105 .
- ↑ a b Todd Werpy, John Frye, John Holladay: Succinic Acid - A Model Building Block for Chemical Production from Renewable Resources. In: Birgit Kamm, Patrisk R. Gruber, Michael Kamm (eds.): Biorefineries - Industrial Processes and Products. Status Quo and Future Directions. Vol. 2. Wiley-VCH Verlag, Weinheim 2006, ISBN 3-527-31027-4 , pp. 367-379.
- ↑ Edzard Scholten, Torsten Renz, Jochen Thomas: Continuous cultivation approach for fermentative succinic acid production from crude glycerol by Basfia succiniciproducens DD1 , in: Biotechnology Letters , 2009 , 31 (12); doi: 10.1007 / s10529-009-0104-4 .
- ↑ Sang Yup Lee, Ji Mahn Kim, Hyohak Song, Jeong Wook Lee, Tae Yong Kim, Yu-Sin Jang: From genome sequence to integrated bioprocess for succinic acid production by Mannheimia succiniciproducens , in: Applied Microbiology and Biotechnology , 2008 , 79 ( 1), pp. 11-22; doi: 10.1007 / s00253-008-1424-3 .
- ↑ a b c I. Bechthold, K. Bretz, p Kabasci, R. Kopitzky, A. Springer: Succinic Acid: A New Platform for Biobased Chemical Polymers from Renewable Resources , in: Chemical Engineering & Technology , 2008 , 31 (5 ), Pp. 647-654; doi: 10.1002 / ceat.200800063 .
- ↑ AM Sanchez, GN Bennett, KY San: Novel pathway engineering design of the anaerobic central metabolic pathway in Escherichia coli to increase succinate yield and productivity , in: Metabolic Engineering , 2005 , 3 , pp. 229-239; doi: 10.1016 / j.ymben.2005.03.001 .
- ↑ a b Keyword Succinic Acid , in: Hans Zoebelein (Ed.): Dictionary of Renewable Resources. 2nd edition, Wiley-VCH, Weinheim and New York 1996, ISBN 3-527-30114-3 , p. 92.
- ↑ James B. McKinley, C. Vieille, J. Gregory Zeikus: Prospects for a bio-based succinate industry , in: Applied Microbiology and Biotechnology , 2007 , 76 (4), pp. 727-740; doi: 10.1007 / s00253-007-1057-y .
- ↑ T. Werpy, G. Petersen: Top Value Added Chemicals from Biomass. Volume I - Results of Screening for Potential Candidates from Sugars and Synthesis Gas. Produced by the Staff at Pacific Northwest National Laboratory (PNNL); National Renewable Energy Laboratory (NREL), Office of Biomass Program (EERE), 2004 ( PDF ).
- ^ Joseph J. Bozell, Gene R. Petersen: Technology development for the production of biobased products from biorefinery carbohydrates - the US Department of Energy "Top 10" revisited , In: Green Chemistry , 2010 , 12 (4), p. 539– 554; doi: 10.1039 / B922014C .




