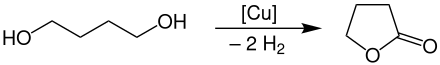γ-butyrolactone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | γ-butyrolactone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 6 O 2 | ||||||||||||||||||
| Brief description |
colorless liquid with a faint odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 86.09 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.13 g cm −3 |
||||||||||||||||||
| Melting point |
−44 ° C |
||||||||||||||||||
| boiling point |
204-206 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
completely miscible with water and ethanol |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−420.9 kJ / mol (l) |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
γ-Butyrolactone (according to IUPAC nomenclature: Oxolan-2-one , often abbreviated as GBL ) is an organic-chemical compound from the group of lactones , more precisely it is the lactone of γ-hydroxybutyric acid (GHB). It is mainly used as a solvent in industry and as a raw material for the manufacture of pharmaceuticals and chemicals . In addition, it serves as a precursor to the narcotic drug GHB ; In Europe and the USA, the issue of GBL is therefore monitored by what is known as monitoring (name for voluntary self-regulation by dealers and manufacturers).
Occurrence
In nature, GBL occurs in traces e.g. B. as a remodeling product of the natural GHB and also in wine. It is industrially synthesized from succinic acid or BDO .
Extraction and presentation
γ-Butyrolactone is produced on an industrial scale by the dehydrocyclization of 1,4-butanediol at temperatures of 180-300 ° C. under normal pressure on copper contacts as a catalyst .
The yield from this process is approximately 95%. The product can be obtained relatively easily by separation from the hydrogen gas phase .
This process is mainly carried out by BASF , Ashland and LyondellBasell .
In addition, GBL can also be synthesized by internal esterification of γ-hydroxybutyric acid in an acidic medium. Conversely, γ-hydroxybutyric acid is produced by saponifying GBL in a basic medium.
properties
Physical Properties
GBL is a colorless liquid at room temperature with a faint odor.
The melting temperature of γ-butyrolactone is −43.5 ° C, the boiling point is 206 ° C. Together with water vapor , GBL is volatile at temperatures that are below the actual boiling point of GBL. The flash point and ignition temperature are 100 and 455 ° C, respectively. The density of GBL is 1.128 g / cm 3 .
Γ-Butyrolactone is soluble in any ratio with water , methanol , ethanol , acetone , ether , dichloromethane , toluene and propylene glycol . However, the solubility of GBL in aliphatic hydrocarbons is very limited.
The viscosity of GBL is relatively low and comparable to water .
Chemical properties
GBL reacts slightly acidic by partial hydrolysis to γ-hydroxybutyric acid in water. The substance is quantitatively hydrolyzed by aqueous alkalis. Anhydrous bases can abstract a proton on the C2 atom (CH-acidic compound, formation of the enolate ), which is then accessible to nucleophilic attack.
GBL, like ethyl acetate or acetone, is a polar aprotic solvent and dissolves many plastics such as polyester or PMMA .
Pharmacokinetics
GBL is hydrolyzed to GHB in human blood by 1,4-lactonase . The resorption of GBL occurs when taken orally faster than GHB, so that the plasma levels of GHB when taking GBL rises faster than taking GHB itself. The plasma half-life of GBL is due to rapid metabolism to GHB less than 60 s, the This means that five minutes after taking GBL, about 3% of the γ-butyrolactone is still present in the body. GHB is primarily metabolized in the body by alcohol dehydrogenase (ADH) / aldehyde dehydrogenases (ALDH) to succinic acid, which in turn passes into the citric acid cycle . Ultimately, only carbon dioxide and water remain as degradation products . To a small extent, metabolites are also formed by β-oxidation , which are excreted renally .
use
Industry
GBL is a widely used solvent in the industry and is also used as a paint remover , graffiti remover , detergent, and nail polish remover . It is contained as a plasticizer in soft PVC films and as an electrolyte component in electrolytic capacitors .
In addition, GBL also serves as a raw material for the production of pharmaceuticals and chemicals for agriculture.
GBL is irreplaceable to the chemical industry, which is the main reason why, unlike GHB, it is not classified as an illegal narcotic . GBL has also been used as a drug since the ban on GHB (Liquid Ecstasy) and also serves as a raw material for the production of GHB.
In November 2007, GBL hit the headlines as part of a plasticine for children. By swallowing the modeling clay, some children allegedly fell "into a narcotic state", i. that is, they felt the effects of GBL as a sleep aid .
drug
GBL is also known as a party drug because it is converted into GHB , which is also known as a drug, in the human body within a few seconds . The former especially after the acquisition of GHB - for example in Germany from 2002 - became legally more difficult. While GHB originally had the scene names Liquid Ecstasy, Liquid E or simply G (pronounced in English), GBL is now also called that because this substance is usually consumed effectively. In the drug and addiction report of the federal government , GBL is always only mentioned together with GHB under the term “ GHB / GBL ”.
GBL has almost the same effect as GHB, but from the point of view of the consumer there are some differences before the effect occurs:
- Compared to GHB, GBL must be dosed significantly lower (about a factor of 0.5) in order to develop the same (GHB) effect. A typical (party) dose for GBL is therefore usually 1.0 to a maximum of 2.0 ml.
- GBL causes a faster onset of action compared to GHB (see above section Pharmacokinetics ).
- GBL must be diluted more strongly (see section Damage through continuous consumption ).
- GBL has a stronger taste of its own, which is often described as sour, while GHB has a rather low taste.
For more information on the effect and detection as a drug, see main article GHB .
Risks
Damage from continuous consumption
Undiluted GBL consumption by humans affects the esophagus , gastric mucosa , colon and small intestine cells , and wound healing can also be delayed. Undiluted GBL also dissolves calcium from the tooth enamel . Drug advice centers and safer use initiatives therefore usually recommend a dilution of 1: 100 to 1: 300 (1 ml GBL to 100–300 ml alcohol-free liquid). Another danger in this context is contamination that may be contained in the non-analytically pure industrial GBL.
Mixed consumption and overdose
Since GBL is metabolized to GHB in the human body within seconds, all risks for the consumption of GHB also apply in principle to the consumption of GBL, in addition to the inherent risks of GBL (see previous section Damage from constant consumption ). An overdose can also be brought about faster with GBL, since GBL can only be dosed about half as much as GHB if one wants to achieve the same (GHB) effect.
For more information on the risks as a drug, see the GHB main article in the section of the same name.
toxicity
The toxicity of GBL has been researched in great detail due to its diverse uses in the chemical industry, including in the production of food. Animal experiments have shown that GBL is metabolized to GHB within a few minutes and excreted as CO 2 with the breath within two to three hours . For mice, the mean lethal dose for a single administration of GBL is in the range between 0.5 and 1.8 g per kilogram of body weight. Administration to animals in smaller doses lasting several months was withstood without damage. GBL is neither genotoxic nor carcinogenic . However, if overdosed, GBL can be fatal (see above).
safety instructions
GBL is a substance which irritates the mucous membranes and is harmful to health. Therefore safety glasses and protective gloves must be worn when working with GBL . Clothing contaminated with GBL must be removed immediately. In addition, good ventilation must be ensured when working with GBL. After skin contact, the affected areas must be washed off thoroughly with soap and water, but the skin must not be scrubbed. After eye contact, the affected eye must be rinsed with running water for at least 15 minutes and then an ophthalmologist should be consulted. If GBL is accidentally swallowed, the mouth must be rinsed with water immediately and plenty of water drunk.
Burning GBL can be extinguished with water , dry chemical , foam and carbon dioxide using chemical protective clothing. Contaminated fire fighting water must be collected separately and must not enter the sewage system.
GBL must be stored in a well-ventilated place and separated from alkalis and base-forming substances.
Legal situation
Germany
GBL is not listed in the Narcotics Act, but Europe relies on voluntary control of the supply by the distributors ( monitoring ). Possession is not a criminal offense, but is regulated by the Chemicals Act and the GefStoffV . The abuse of GBL for the synthesis (production) of GHB is punishable . In recent years, due to the ban on GHB, an increase in GBL consumption has been observed.
GBL may fall under the legal definition of Section 2 (1) AMG . Then manufacture and sale are regulated according to the AMG, regardless of the form in which it is available, and require approval. This was confirmed in a ruling by the Federal Court of Justice .
The extent to which this decision is tenable after the judgment of the European Court of Justice on July 10, 2014 is legally controversial. The ECJ ruled that the AMG can only be used if the substance concerned is a medicinal product and not substances "which are only consumed to induce intoxication and which are harmful to health". Current comments on the Medicines Act reflect this legal situation. However, there is currently no recent decision by German federal courts with regard to GBL.
Australia
In Australia, GBL is a border controlled substance and importation without a permit is illegal.
Canada
GBL is a Controlled Substance in Canada under Schedule VI of the Controlled Drugs and Substances Act.
Hong Kong
In Hong Kong GBL is treated as dangerous drug controlled under Schedule 1 according to the Dangerous Drugs Ordinance, Cap.134 with exceptions according to Paragraph 16D.
Israel
GBL has been classified as a prescription only in Israel since 2007.
Netherlands
GBL is freely available as a cleaning agent in the Netherlands.
Sweden
GBL is classified as a hazardous substance in Sweden and has been a controlled substance since 2011 .
Switzerland
GBL is a narcotic in Switzerland, provided the substance is not used industrially. As with any other narcotic, private use is a criminal offense.
United Kingdom
According to regulation 4B of 2001, the import, export, manufacture, distribution and possession of GBL and 1,4-BD is permitted, provided that it is not intended for human consumption.
United States
GBL is classified as List 1 controlled chemical in the USA . As an analog of GHB, it is a Schedule I controlled substance under the Controlled Substances Act if it is used for human consumption.
Individual evidence
- ↑ a b c d e Entry on γ-butyrolactone in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c d data sheet γ-butyrolactone (PDF) from Merck , accessed on January 19, 2011.
- ↑ a b Entry on gamma-butyrolactones in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on April 23, 2016.
- ↑ a b CRC Handbook 90th edition (2009–2010), pp. 5–26 ( Memento of April 26, 2015 in the Internet Archive ). - See also entry on butyrolactones . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed March 1, 2016.
- ↑ J. Vose, T. Tighe, M. Schwartz, E. Buel: Detection of gamma-butyrolactone (GBL) as a natural component in wine . In: Journal of Forensic Sciences . tape 46 , no. 5 , 2001, p. 1164-1167 , PMID 11569560 .
- ^ A b c Wolfgang Schwarz, Jürgen Schossig, Roland Rossbacher, Rolf Pinkos, Hartmut Höke: Butyrolactone. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley ‐ VCH Verlag GmbH & Co. KGaA., May 17, 2019, p. 2f., Doi : 10.1002 / 14356007.a04_495.pub2 .
- ↑ The Drugs Commissioner of the Federal Government: Drugs and Addiction Report May 2015. Online under Archived Copy ( Memento of the original from July 22, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , accessed September 15, 2015
- ↑ http://www.drogen-info-berlin.de/htm/ghb.htm
- ↑ https://drugscouts.de/de/lexikon/gbl
- ↑ NTP Study Reports TR-406: Toxicology and Carcinogenesis Studies of γ-Butyrolactone (CAS No. 96-48-0) in F344 / N Rats and B6C3F1 Mice (Gavage Studies) or Cognitive Enhancement Research Institute Discussion .
- ↑ a b BGH , judgment of December 8, 2009, Az. 1 StR 277/09, full text .
- ↑ Erwin Deutsch, Hans-Dieter Lippert (Ed.): Commentary on the Medicines Act (AMG). Springer, 2010, pp. 64–66 ( limited preview in Google book search).
- ↑ § 2 Paragraph 1 No. 5 old version, § 2 Paragraph 1 No. 2a new version, § 5 , § 95 Paragraph 1 No. 1.
- ↑ a b Criminal law: trading in gamma-butyrolactone (GBL; liquid ecstasy) for consumption purposes
- ↑ ECJ, judgment of July 10, 2014, Az. C-358/13, C-181/14, C-358/13, C-181/144, full text .
- ^ Kügel, Müller, Blattner: Medicines Act, Commentary ; 2nd edition, 2016; CH Beck Verlag; Marg. 86, para. 99
- ↑ LAW AND JUSTICE LEGISLATION AMENDMENT (SERIOUS DRUG OFFENCES AND OTHER MEASURES) ACT 2005 NO. 129, 2005 - SCHEDULE 1 .
- ^ Controlled Drugs and Substances Act (SC 1996, c. 19) .
- ↑ section 7c of chapter B of part A of the 1st appendix of the Dangerous Drugs Act 1973 .
- ↑ Verkopers schoonmaakmiddel earn fors aan partydrug GHB ( Dutch ) In: Trouw . April 11, 2012. Retrieved April 11, 2012.
- ↑ Socialutskottets betänkande 2010/11: SoU5 - Riksdagen .
- ↑ Decision of the Swiss Federal Court of July 15, 2014 (6B_1067 / 2013) E. 1.5
- ^ A Change to the Misuse of Drugs Act 1971: Control of GBL, 1,4-BD, BZP and related piperazine compounds, a further group of anabolic steroids and 2 non-steroidal agents, synthetic cannabinoid receptor agonists and oripavine
- ↑ UK Statutory Instrument 2011 No. 448 . February 18, 2011. Retrieved January 16, 2015.
- ↑ Information Bulletin: GHB Analogs; GBL, BD, GHV, and GVL .
Web links
- The Emergence of GHB Alternatives (GBL as GHB Alternative-Engl.)
- The Commercial Chemistry of GHB (connection GHB / GBL chemisch-engl.)
- Toxicological assessment of γ-butyrolactone (PDF) at the professional association raw materials and chemical industry (BG RCI), accessed on August 22, 2012.
- Inquiries about GBL- u. GHB poisoning to the Swiss Toxic Center