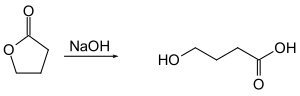
4-hydroxybutanoic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 4-hydroxybutanoic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 4 H 8 O 3 | |||||||||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 104.11 g · mol -1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| Melting point |
−17 ° C |
|||||||||||||||||||||
| boiling point |
178–180 ° C (decomposition) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
4-hydroxybutyric acid or γ-hydroxybutyric acid , short GHB (gamma-hydroxybutyric acid) is a hydroxy - carboxylic acid , their salts as 4-hydroxybutyrate , or in the pharmaceutical industry as oxybate be referred to.
GHB is closely related to the human neurotransmitter GABA (gamma-aminobutyric acid) and is also an independent neurotransmitter in the mammalian body. GHB is manufactured synthetically, used medicinally or as a drug.
history
GHB was first synthesized in 1874 by Alexander Saytzeff , but the pharmacological effect was not discovered until 1960, when the substance was examined by the surgeon Henri Marie Laborit at the naval base in Toulon on behalf of the French Navy as part of a research program . Since the amino group of the GABA molecule has been replaced by a hydroxy group in GHB , the molecule can cross the blood-brain barrier . In the 1960s and 1970s, GHB was used extensively as a narcotic . GHB has also been used as an aid to alcohol withdrawal and as a dietary supplement for athletes. The indication of GHB was initially limited to narcolepsy with cataplexy , later it was extended to the general treatment of narcolepsy.
In the 1980s, GHB became known in the US bodybuilding scene for its alleged anabolic effects (see Doping section ). It has been widespread as a party drug in the USA since the 1990s, and also in Europe from the turn of the millennium.
Chemical properties
4-hydroxybutanoic acid is a moderate to strong acid having a p K S value of 4.4. It cyclizes in an equilibrium reaction , depending on the pH value and the temperature, to γ-butyrolactone (GBL).
The salts of 4-hydroxybutanoic acid are odorless and in some cases hygroscopic . The potassium salt has a very salty taste. Sodium 4-hydroxybutyrate ("sodium oxybate") has a distinctly salty taste.
pharmacology
Pharmacodynamics (mechanism of action)
GHB is a full agonist with nanomolar affinity on the human GHB receptor ( K d = 114 nM). The GHB metabolite trans -4-hydroxycrotonic acid binds to the GHB receptor more strongly than GHB and acts as an agonist.
At extrasynaptic GABA-A receptors of the α4β1δ type, GHB acts as a partial agonist , the mean effective dose (EC 50 ) of which is 140 nM.
Pharmacokinetics
By alcohol dehydrogenase (ADH) or aldehyde dehydrogenases (ALDH) GHB is in the body to succinic acid metabolism, which in turn in the citric acid cycle passes, causing them to carbon dioxide is reduced and water. To a small extent, metabolites are also formed by β-oxidation, which are excreted renally .
GHB is not metabolized to the related GABA in the body , but it does increase the depressant effect of GABA. Furthermore, GHB leads to an increased release of dopamine . The hypothesis according to which GHB first hampers the release of dopamine (which is said to lead to fatigue) and then triggers its increased release (which causes sleep disorders) is unproven.
Compared to other drugs, GHB has a very short half-life, which means that it can be detected for a maximum of twelve hours in the urine and a maximum of eight hours in the blood serum (see section Detection ).
pathology
A succinate semi-aldehyde dehydrogenase deficiency is a metabolic disorder and physiologically leads to an increase in GABA and GHB levels.
Manufacturing
GHB is found in traces in food, e.g. B. in meat, because it is present as an independent neurotransmitter in the mammalian body.
One way of the synthetic production of GHB is the hydrolytic cleavage of the internal ester bond of γ-butyrolactone by the equivalent amount of an alkali metal hydroxide (usually sodium hydroxide ) with initial supply of heat.
Use and effect
Spectrum of activity
Depending on the dosage, GHB acts either as an entactogen , muscle relaxant or as a sleep aid .
The fact that GHB acts as a sleeping aid or narcotic in higher doses , but has a stimulating and mood-enhancing effect in lower doses, is related to the fact that when the GHB receptor is activated, glutamate is released, the most important excitatory neurotransmitter . In larger quantities it activates the GABA B receptor, which leads to the sedative effect. The effect of GHB on dopamine release is two-phase: lower concentrations stimulate dopamine release via the GHB receptor and higher concentrations inhibit release via the GABA B receptor. Both the inhibition and the release of dopamine are prevented by opioid antagonists such as naloxone and naltrexone .
Medical use
GHB is used in medicine as an intravenous anesthetic for caesarean sections , in obstetrics and in all types of risk cases (e.g. patients with liver damage, cardiac catheterization, etc.). 4-Hydroxybutanoic acid sodium salt (sodium oxybate) was approved as a drug for the symptomatic treatment of narcolepsy in the USA in 2002 and in the EU in 2005 . For this it is taken in dissolved form.
At higher doses, GHB has a strong soporific effect. The salts of 4-hydroxybutyric acid are therefore used medically as an alternative anesthetic without analgesic effects. The free 4-hydroxybutyric acid is too reactive (intramolecular esterification to GBL) and strongly acidic.
In Germany, the substance is approved as a (BtM) prescription drug for narcolepsy patients. It becomes effective after several days of use as a narcotic against the cataplexies that often occur in narcolepsy, after a few weeks there is a clear decrease in cataplexies. It is also said to have a positive effect on daytime sleepiness in narcolepsy.
According to current knowledge, GHB does not cross the placenta and can therefore continue to be taken by pregnant narcolepsy patients under medical supervision. Since NaGHB is passed on to the baby in breast milk, breastfeeding should be done early.
GHB is also used to treat Parkinson's disease , because by stimulating the release of dopamine, the deficiency of this neurotransmitter can be reduced. In Austria the substance is used to treat withdrawal symptoms in alcoholics .
Due to some deaths in the United States, the FDA has stepped up contraindications and warnings for GHB. According to the European guidelines, the same applies to a lesser extent. The FDA cites simultaneous treatment with opioid analgesics, benzodiazepines , sedating antidepressants , antipsychotics , general anesthetics and muscle relaxants as relative contraindications . Alcohol consumption is an absolute contraindication.
Party drug
GHB is also used as a party drug around the world . Scene names include Liquid Ecstasy - although it has no chemical relationship to MDMA , which is usually sold as Ecstasy - also based on it Liquid , Liquid E or Liquid X , and Fantasy , Gamma , Soap , Georgia Home Boy , Scoop , Cherry Meth , Blue Nitro or often just G (pronounced English). All these scene names are now also used for the precursor substance GBL .
GHB was legally available to everyone until it was banned - in Germany from 2002. Since then it has been available on the black market as a hygroscopic solid and as a colorless or (with food coloring) colored liquid in bottles, which is an aqueous solution of GHB salts. Since the prohibition of GHB, however, GBL or BDO have been consumed very often . Both substances are metabolized directly to GHB in the body and therefore have almost the same effect - in addition, some of them are legally available. In the drug and addiction report of the federal government, GHB is therefore mentioned almost exclusively combined with GBL under the term “GHB / GBL”. Both GBL and BDO ultimately have the same effect as GHB, but have to be dosed significantly lower (GBL around a factor of 0.5).
The effect of intoxication depends on the dosage and the development of tolerance . In low to medium doses (GHB: about 1.0 ml to 5.0 ml; GBL: about 0.5 ml to 2.5 ml) when taken orally, GHB causes intoxication that is partially similar to alcohol intoxication . Possible desired effects are euphoria or mood improvement and the like. a. Due to fear resolution, increased self-confidence and willingness to take risks, sexual stimulation , strong relaxation of the muscles, more intense emotional perception, increased need for communication up to logorrhea , urge to move, appetite and pain relief . Possible undesirable side effects are dizziness , nausea , drowsiness (see section Risks ), hunger and dilated pupils ( mydriasis ). Cardiovascular problems can also arise as GHB acts on the catecholamine receptors in the brain. Higher dosages (GHB: from about 5 ml; GBL: from about 2.5 ml) lead, depending on the dose, to a sleepy to narcotic condition (see section Risks and Medical Use ). The maximum plasma concentration in humans is already reached after 15–40 minutes, depending on the dose, the duration of the effect of the intoxication is at least 90 to a maximum of 180 minutes.
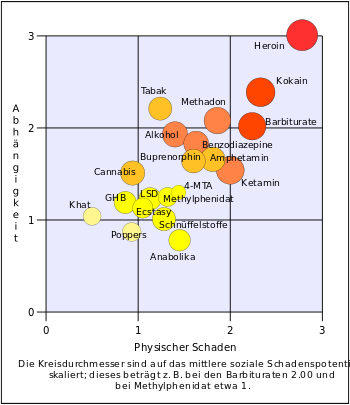
The hazard potential has been investigated more intensively in recent years, for example in 2017 by the Baden-Württemberg State Medical Association. The report, which in turn refers to “essential clinical findings and experiences over the past ten years”, makes the following statement on the potential for addiction : “GBL has a considerable potential for addiction, this has been proven by case reports since 1994”. Even with occasional consumption, acute intoxication can lead to unconsciousness, epileptic seizures and also respiratory depression. Other consequences of the overdose include "cramps, twitching, screaming" and vomiting, whereby an overdose with GHB / GBL occurs more easily than with 'conventional' party drugs such as amphetamine and MDMA, for example. This can be explained by the fact that the dosage steps are extremely staggered (cf. medium dose GBL: 1–1.4 ml, high dose: 1.5–2 ml, narcotic dose:> 2 ml) and the measurement of this dose is also included Increasing intoxication made more difficult: "Such overdoses happen because GBL users sometimes forget that they have already taken something shortly before". These consequences of the overdose have not only individual consequences, but also social ones, as people in the vicinity of the overdosed person have to provide help. Sexually unrestrained behavior can also be perceived as extremely unpleasant. Furthermore, "the most severe withdrawal courses are possible". These can range up to delirium. In Internet forums such as drug scouts , numerous reports from people can be found who describe the most severe course of withdrawal. This is confirmed by scientific findings from 2018, for example from the physician Felix Betzler
The withdrawal of GHB / GBL is now, otherwise even considered yet by David Nutt in 2007 more difficult than withdrawal from heroin. In addition, the relapse potential is very high (see addiction and withdrawal).
According to official figures, the spread of GHB as a party drug is very low compared to other relevant substances. The European Drug Report 2015 refers to a partial evaluation of the Global Drug Survey , a non-representative online survey, according to which among 25,790 people between the ages of 15 and 34 in ten European countries who regularly take part in "club events" received a 12 -Monthly prevalence for GHB of 2%. Even if this number is not representative, it is well below the values measured in the same analysis for other drugs (cannabis 55%, ecstasy 37%, cocaine 22%, amphetamines 19%, ketamine 11%, mephedrone 3%, synthetic cannabinoids 3%), although it is unclear to what extent a stylization of GHB as a date rape drug (see section knockout drops ) influences the response behavior.
Recent observations suggest a different picture. In Berlin, at least one patient with suspected GHB / GBL intoxication comes to a hospital every weekend.
GHB, or the precursor substance GBL , which is converted to GHB in the body, is not tolerated in most cases due to the high risk potential , even in places that tolerate drug use to a certain extent. In the past , the Fusion Festival explicitly warned against GHB in the program booklet, in the Berlin techno club Wilde Renate there is a sign in the entrance area with the inscription "No GHB - otherwise Spree", in the KitKatClub there is also a corresponding notice.
Ko drops
Since around 2004, GHB has been mentioned several times in press reports as a so-called rape drug or knockout drop , although there is no scientifically sound study that proves the dominance of GHB in such offenses, but rather data that speak against it (see below) . A well-known criminal case in which GHB was used is the case of the millionaire heir Andrew Luster . This and other cases in the US and Japan led US media to stylize GHB as a date rape drug . Since 2008 the Bavarian State Criminal Police Office has also reported that GHB is used to mostly drugged women in discos and to sexually abuse or rob them.
A study carried out in Great Britain and published in 2006, for which 120 suspected cases of the presumed use of knockout drops were examined, initially only came to the result that in ten cases (8.3%) the secret administration of a corresponding drug was carried out at all Means could be proven. However, the consumption of GHB could only be proven in two of the total of 120 suspected cases (corresponds to 1.7%), although it is unclear whether GHB was secretly administered here. Thus, in at least 80% of the proven cases of the use of knockout drops instead of GHB, another anesthetic substance was detectable. Further results of the study were that 119 of the 120 alleged victims had drunk alcohol (99.2%), in 22 cases an alcohol content of at least 2 g / l was found in the blood (18.3%). Cannabis (20%) and cocaine (17%) were also significantly more represented than GHB among all suspected cases.
If GHB is used as Ko-Drops , an effect in the form of drowsiness or comatose sleep can occur within 15–30 minutes - but only with the corresponding high dosage. Potential perpetrators are thus faced with the "challenge" of precisely dosing an attack, taking into account the victim's condition, since the effects desired by voluntary users such as the urge to move and euphoria outweigh the lower doses Related to alcohol. In the case of a correspondingly high dosage, the memory of the time under the influence of drugs is sometimes only incomplete ( anterograde amnesia , " Halcion effect"). The most commonly used sodium salt has a distinctly salty to soapy taste. Concrete proof of such incidents is difficult, as GHB can only be detected in special laboratories and within a short time window (see section Evidence ).
In the discourse, however, the opposing position speaks of a “witch hunt”. The taz commented that the problem was “the secrecy, the assault and the rapist - and not the material”. The Frankfurter Allgemeine Zeitung writes that most stories about anesthetic drops are “nothing more than modern sagas”.
doping
In the 1980s, the substance was used by athletes as a doping agent, because on the one hand it is supposed to release increased growth hormones and on the other hand it is supposed to ensure restful sleep.
Risks
Mixed consumption
Mixed consumption of GHB and other substances is particularly dangerous compared to many other drugs and essentially involves two basic areas of danger:
- Nausea and vomiting , which, in connection with the potentially narcotic properties of the substance , can lead to death by suffocation at high doses . This is especially the case when combined with alcohol and milk or milk products .
- Bradycardia , respiratory paralysis or respiratory depression and / or circulatory collapse , especially when combined with alcohol or drugs that have a respiratory depressive effect (e.g. benzodiazepines ), antihistamines , antiretroviral drugs (protease inhibitors, e.g. Crixivan, Invirase, Kaletra, Norvir, Reyataz), Ketamine , opioids (e.g. heroin , methadone , polamidon ), sexual enhancers such as Viagra or poppers .
Caffeine is considered to be a natural blocker of the GHB effects, which - albeit only to a very limited extent - should provide the opportunity to force disillusionment. On the other hand, previously consumed caffeine initially blocks the full development of its effects and can thus lead to an overdose by the consumer. The latter also applies to the combination with speed .
Overdose
In higher doses, restrictions in motor control, similar to those associated with heavy alcohol consumption , can occur.
GHB overdoses (i.e., doses that result in undesirable anesthesia) are life-threatening when taken alone because of the risk of respiratory depression and apnea. This applies to an even greater extent in combination with other central depressant drugs such as sleeping pills, opioids or depressant drugs such as alcohol and heroin .
Because of the soporific effect at high doses, the condition of the affected person can initially be misjudged by healthcare professionals. Usually an overdose of benzodiazepines is suspected, so that symptomatic intensive care treatment (possibly also the additional administration of flumazenil ) is undertaken.
After a sleep induced by GHB, you wake up suddenly after a certain time (depending on how high the dose was) - provided no further complications (e.g. vomiting) have occurred , because over time the concentration of GHB falls below the activation threshold the GABA B -receptors decreases and primarily the GHB-receptors are activated again, which has a stimulating effect (" stand-up man effect").
Deaths from mono-consumption of GHB have so far only occurred sporadically in the case of extreme overdoses and treatment errors. However, mixed consumption with drugs with a central depressant effect is particularly dangerous . B. alcohol , heroin .
Impurities
As with all other illegal drugs, GHB also runs the risk of consumers receiving a contaminated product on the black market, with a theoretically unlimited risk spectrum.
Addiction and withdrawal
"GBL has a considerable potential for addiction, this has been proven by case reports since 1994". This is also confirmed by Peter Neu, the chief physician at the Clinic for Psychiatry and Psychotherapy at the Jewish Hospital Berlin.
As with the drugs mentioned, GHB also has a certain potential for psychological dependence , i. In other words , the user may feel the desire - especially after a trip that is perceived as positive - to repeat the drug use or automatically link the use to certain behaviors, such as going to parties. Often GHB (or the precursor substance GBL) is then regularly consumed in situations in which drinking alcohol for intoxication is to a certain extent socially acceptable.
Much more problematic, however, is the potential for physical dependence that is also present in GHB . In such a case, withdrawal symptoms arise after discontinuation , which qualitatively resemble those of benzodiazepines , but do not reach the extent in intensity and duration (twelve to 96 hours). Physically , the following characteristic symptoms can then occur: enormous sweating , muscle tremors , psychomotor restlessness up to epileptic seizures, accompanied by other symptoms such as diarrhea or nausea . Psychologically , it can lead to aggressiveness and insomnia , which, if predisposed, are accompanied by acute psychotic symptoms ( delusions , hallucinations , depersonalization, derealization , sometimes also dissociation ). Depending on the intensity, these symptoms then require clinical help (see section on emergency therapy ).
Risks of the precursors
Since drug users who want to consume GHB nowadays in fact use GBL in particular, which is metabolized directly to GHB in the human body, in practice the GBL-specific risks also play a role in addition to the GHB-specific risks. The risks that arise from insufficient dilution should be emphasized here.
For further information on GBL-specific risks, see the same section in the main article GBL .
Other possible risks
So far there is little evidence that people are at risk of further long-term (organ) damage through GHB consumption.
A report by the European Monitoring Center for Drugs and Drug Addiction , which was published in 2008 under the heading GHB and its precursor GBL: an emerging trend case study , does not list anything in the chapter on negative health consequences . On the other hand, the potential for addiction, side effects such as headaches and sleep disorders as well as the number of emergency services due to the risks mentioned (e.g. mixed consumption and overdosing) are mentioned.
A publication by the State Medical Association of Baden-Württemberg lists the following as special dangers: 1. Acute intoxication , v. a. in the case of high consumption, lack of tolerance formation, high degree of purity and / or mixed consumption with alcohol / benzodiazepines (see sections mixed consumption , overdose ). 2. Dependency : This should be assumed after the development of tolerance and then regular consumption several times a day (see section Dependence and withdrawal ).
A study at the University of Malaga , published in the International Journal of Neuropsychopharmacology in 2009, concluded that the administration of low doses (10 mg / kg) of GHB in rats led to neurological deficits, such as the grasping reflex and spatial awareness and working memory . Deficits were observed in the CA1 area of the hippocampus and in the prefrontal cortex . When higher doses (100 mg / kg) were administered, the effects were not as measurable for reasons that were not explained. It is unclear to what extent the results can be transferred to humans.
Deaths
According to Hilke Andresen-Streichert, who heads the forensic toxicology department at Cologne University Hospital, the number of unreported deaths is probably high.
Deaths proven to be due to GHB use are rather rare , according to the report by the European Monitoring Center for Drugs and Drug Addiction, published in 2008 under the heading GHB and its precursor GBL: an emerging trend case study . Presumably since the substance appeared in the 1990s until the time the report was drawn up (2008), the observatory reported one death each from Denmark and Italy, two from Finland and three each from Norway and Great Britain, which, however, was reported the survey methods can be attributed. In Sweden, where a more detailed study on the subject was carried out, a total of 36 deaths were counted between 1996 and 2004. Most of these cases have been classified as accidental poisoning (accidental poisoning) or suicides (suicides).
According to the report, an unpublished study from Great Britain lists a total of 44 deaths that can be attributed to GHB or GBL for the period from 1995 to 2006 - in opposition to the reported number of cases (see above). In most of these cases, "at least two other substances were consumed, typically alcohol and / or stimulants," it says. Typical hospital emergency situations are coma , bradycardia, and hypothermia , according to the report .
In 2014, the Federal Criminal Police Office in Germany registered a total of twelve deaths (2013: nine cases) that were related to the consumption of GHB or GBL. There were five in 2015, four in 2015 and seven in 2017. For example, the case of a 17-year-old from Lingen made headlines in 2015 , who, according to the press report, had apparently drunk “rim cleaner” - for which the GHB precursor GBL is also used - without any drug experience. In another case, a 27-year-old in Bamberg had drunk from a “plastic bottle with the dangerous gammabutyrolactone” at a party and died of an overdose. A later convicted 24-year-old had left the bottle at the party and warned of the dangers, but the warning was apparently not understood by the drunk party guests.
Emergency therapy
In cases of acute intoxication, for example due to overdose or mixed consumption (see above), strict inpatient intensive clinical monitoring with readiness for intubation and oxygen administration and otherwise symptomatic treatment is generally required. Neuroleptics must not be used during intoxication . The possibility of antagonization with physostigmine is controversial.
In the case of treatment of withdrawal symptoms after a developed physical dependency, acute intoxication must first be ruled out because of possible intolerance of the drugs to be administered with GHB (see section mixed consumption ). If this can be ruled out, benzodiazepines are administered (up to 60 mg diazepam per day) and, if necessary, additional antipsychotics ( olanzapine up to 20 mg / day or Haldol up to 15 mg / day). In the case of vital danger, there is an intensive obligation, u. U. with administration of propofol , barbiturates and / or clomethiazole .
proof
The detection time of GHB in the blood is around 6–8 hours, in the urine around 12 hours. Evidence in urine and blood serum can only be provided by complex and sensitive measurement processes in special laboratories using GC-MS (coupling a gas chromatograph (GC) with a mass spectrometer (MS)) due to the extensive metabolism of GHB to carbon dioxide and water . Since 2010 there has been an enzymatic GHB direct detection in serum and urine. The physiologically occurring GHB can be distinguished from exogenous consumption by the corresponding limit value (<4 / <6 μg / ml).
A blood sample should be at least 2 ml, better 10 ml, without added citrate. A urine sample - which is more sensible anyway due to the longer time window - should contain approx. 100 ml.
In the case of suspected secret administration of GHB or GBL, victim counseling centers recommend taking a urine sample within 12 hours of the presumed admission, recording the time it was taken and keeping the sample refrigerated, unless a doctor can be consulted during this time. A sample taken in this way may have U. no legal evidential value, but can at least provide the alleged victim with certainty.
Proof of a hair sample is also theoretically possible . On the one hand, a prerequisite is a corresponding hair length at the time of sampling. On the other hand, however, a one-off exposure in this way can hardly be proven, since a distinction between endogenous and endogenous plus uniquely exogenous occurrence can hardly be achieved using hair analysis. However, multiple consumption can still be proven in this way over a longer period of time.
Legal situation
With the 16th ordinance amending the provisions of the law on narcotics dated November 28, 2001, which came into force on March 1, 2002, GHB was classified as a narcotic in Germany. Dealing with GHB is not permitted outside of the medically approved area. Since then, GHB has been listed in Appendix III to § 1 BtMG (marketable and prescription substances). Injectables like Somsanit are exempt from the requirements of the Narcotics Prescription Ordinance; they are subject to simple prescription requirements.
In Switzerland, GHB has been subject to narcotics law since January 1, 2002.
In Austria, too, GHB was included in the Narcotics Act in 2002. Therefore, apart from the medical field of application, any possession, trade, import or export is punishable and is punishable by fines or imprisonment.
Trade names
Alcover (A, I), Somsanit (D), Xyrem (D, A, CH)
reception
GHB plays a central role in Tatort, which was broadcast for the first time in 2015 : The Last Wiesn .
literature
- H. Andresen, T. Stimpfl, N. Sprys, T. Schnitgerhans, A. Müller: Liquid Ecstasy - a relevant drug problem. In: Dtsch. Medical journal. 105 (36), 2008, pp. 599-603.
- G. Galloway, S. Frederick, F. Staggers, M. Gonzales, S. Stalcup, D. Smith: Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence. In: Addiction. 92 (1), 1997, pp. 89-96.
- J. Hillebrand, D. Olszewski, R. Sedefov: GHB and its precursor GBL: an emerging trend case study . EMCDDA thematic paper. Lisbon, 2008, ISBN 978-92-9168-314-7 .
- B. Luck, L. Afflerbach, H. Graß: Sexualized violence. How the suspicion of a "knockout drop" can be proven. In: Dtsch. Medical journal. 105 (7), 2008, pp. 287-288.
- K. Miotto, B. Roth: Emerging trends in GHB withdrawal syndrome, detoxification . (PDF) 2001.
- Michael Rath, M. Leibfahrt: Statement to the addiction committee of the Federal Directors' Conference on the current GHB / GBL problem. 2009.
- Michael Rath: Results of the survey on GBH / GBL. Lecture at the annual meeting of the Addiction Committee of the Federal Directors' Conference of Psychiatric Hospitals, 2010.
- Snead OC, Gibson KM: gamma-Hydroxybutyric acid. In: N. Engl. J. Med. , Vol. 352, 2005, pp. 2721-2732. PMID 15987921 .
- G. Trendelenburg, A. Ströhle: Gamma-hydroxybutyric acid neurotransmitter, medicament and drug. In: The neurologist. 76 (7), 2005, pp. 832, 834-838.
- Ward Dean, John Morgenthaler, Steven Fowkes: GHB: The Natural Mood Enhancer. Smart Publications, Petaluma CA 1998.
Web links
- GHB . In: Erowid . (English)
- Gamma Hydroxybutyrate (GHB) fact sheet . (PDF) Federal Office of Public Health FOPH
- Gamma-Hydroxybutyrate Toxicity
- Information on Xyrem. ( Memento of April 29, 2008 in the Internet Archive ) FDA (English)
- Metabolism and GBL as an alternative drug use (English)
Individual evidence
- ↑ a b entry on hydroxybutyric acids. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- ↑ M. Sylvia Stein: Statement on the non-small amount of γ-hydroxybutyric acid. In: Toxichem. Krimtech. Volume 70, No. 2, 2003, pp. 87-92. gtfch.org (PDF; 58 kB)
- ↑ MR Witkowski et al. a .: GHB free acid: II. Isolation and spectroscopic characterization for forensic analysis. In: J. Forensic. Sci. Volume 51, 2006, pp. 330-339. PMID 16566766 , doi: 10.1111 / j.1556-4029.2006.00074.x .
- ↑ There is not yet a harmonized classification for this substance . What is shown is a labeling of CAS no. Derived from a self-classification by the distributor . 591-81-1 in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on June 19, 2018.
- ↑ Entry on 4-hydroxybutanoic acid in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Alexander Saytzeff: About the reduction of succinyl chloride. In: Liebig's annals of chemistry. Volume 171, 1874, pp. 258-290. doi: 10.1002 / jlac.18741710216 ( free full text - Internet Archive ).
- ↑ LA Ciolino et al. a .: The chemical interconversion of GHB and GBL: forensic issues and implications. In: J. Forensic. Sci. Volume 46, 2001, pp. 1315-1323. PMID 11714141 .
- ↑ SA Hennessy et al. a .: The reactivity of gamma-hydroxybutyric acid (GHB) and gamma-butyrolactone (GBL) in alcoholic solutions. In: J. Forensic. Sci. Volume 49, 2004, pp. 1220-1229, PMID 15568693 ( full text (PDF)).
- ↑ External identifiers or database links for sodium 4-hydroxybutyrate : CAS number: 502-85-2, EC number: 207-953-3, ECHA InfoCard: 100.007.231 , PubChem : 23663870 , ChemSpider : 9983 , DrugBank : DB09072 , Wikidata : Q7553347 .
- ↑ C. Andriamampandry, O. Taleb, V. Kemmel, JP Humbert, D. Aunis, M. Maitre: Cloning and functional characterization of a gamma-hydroxybutyrate receptor identified in the human brain . In: FASEB J . tape 21 , no. 3 , 2007, p. 885 , PMID 17197387 .
- ↑ M. Maitre, C. Klein, AG Mensah-Nyagan: Mechanisms for the Specific Properties of γ-Hydroxybutyrate in Brain . In: Med Res Rev . tape 36 , no. 3 , 2016, p. 363 , PMID 26739481 .
- ↑ P. Wellendorph, S. Høg, JR Greenwood, A. de Lichtenberg, B. Nielsen, B. Frølund, L. Brehm, RP Clausen, H. Bräuner-Osborne: Novel cyclic gamma-hydroxybutyrate (GHB) analogs with high affinity and stereoselectivity of binding to GHB sites in the rat brain . In: J. Pharmacol. Exp. Ther. tape 315 , no. 1 , 2005, p. 346-351 , doi : 10.1124 / jpet.105.090472 , PMID 16014570 .
- ↑ N. Absalom, LF Eghorn, IS Villumsen u. a .: α4βδ GABAA receptors are high-affinity targets for γ-hydroxybutyric acid (GHB) . In: Proc. Natl. Acad. Sci. USA . 2012, doi : 10.1073 / pnas.1204376109 , PMID 22753476 .
- ↑ F. de Feudis, B. Collier: Amino acids of brain and gamma-hydroxybutyrate-induced depression. In: Arch Int Pharmacodyn Ther. 187, 1970, pp. 30-36.
- ^ MP Castelli, L. Ferraro, I. Mocci et al. a .: Selective gamma-hydroxybutyric acid receptor ligands increase extracellular glutamate in the hippocampus, but fail to activate G protein and to produce the sedative / hypnotic effect of gamma-hydroxybutyric acid . In: J. Neurochem. tape 87 , no. 3 , November 2003, p. 722-732 , doi : 10.1046 / j.1471-4159.2003.02037.x , PMID 14535954 .
- ↑ N. Dimitrijevic, S. Dzitoyeva, R. Satta, M. Imbesi, S. Yildiz, H. Manev: Drosophila GABA (B) receptors are involved in behavioral effects of gamma-hydroxybutyric acid (GHB) . In: Eur. J. Pharmacol. tape 519 , no. 3 , September 2005, pp. 246-252 , doi : 10.1016 / j.ejphar.2005.07.016 , PMID 16129424 .
- ↑ M. Maitre, V. Hechler, P. Vayer and a .: A specific gamma-hydroxybutyrate receptor ligand possesses both antagonistic and anticonvulsant properties . In: J. Pharmacol. Exp. Ther. tape 255 , no. 2 , November 1990, pp. 657-663 , PMID 2173754 .
- ^ I. Smolders, N. De Klippel, S. Sarre, G. Ebinger, Y. Michotte: Tonic GABA-ergic modulation of striatal dopamine release studied by in vivo microdialysis in the freely moving rat . In: Eur. J. Pharmacol. tape 284 , no. 1-2 , September 1995, pp. 83-91 , doi : 10.1016 / 0014-2999 (95) 00369-V , PMID 8549640 .
- ↑ M. Mamelak: Gammahydroxybutyrate: an endogenous regulator of energy metabolism . In: Neurosci Biobehav Rev . tape 13 , no. 4 , 1989, pp. 187-198 , doi : 10.1016 / S0149-7634 (89) 80053-3 , PMID 2691926 .
- ↑ a b Hansjörg Lammers: Gammahydroxybutyric acid GHB: Discover the rejuvenating effect and the regenerative success (part 2) . ( Memento of December 3, 2012 in the Internet Archive ; PDF; 2.77 MB) In: COmed. Volume 7, 2004, pp. 1-3.
- ↑ FDA Drug Safety Communication: Warning against use of Xyrem (sodium oxybate) with alcohol or drugs causing respiratory depression. ( Memento from June 3, 2015 in the Internet Archive )
- ↑ D. Nutt, LA King, W. Saulsbury, C. Blakemore: Development of a rational scale to assess the harm of drugs of potential misuse . In: The Lancet . tape 369 , no. 9566 , March 24, 2007, p. 1047-1053 , doi : 10.1016 / S0140-6736 (07) 60464-4 , PMID 17382831 .
- ↑ GHB facts . Australian Drug Foundation; updated March 25, 2015, accessed September 29, 2015.
- ↑ Drug Commissioner of the Federal Government (Ed.): Drugs and Addiction Report 2015 . Berlin May 2015 ( full text [PDF; 9.1 MB ; accessed on June 19, 2018]).
- ↑ a b Toxicological assessment of γ-butyrolactone (PDF) at the professional association raw materials and chemical industry (BG RCI), accessed on August 22, 2012.
- ↑ a b c d e f g h Friedemann Hagenbuch: GBL / GHB - still the new kick? The most important things for practice at a glance. (PDF; 89 kB) Baden-Württemberg State Medical Association , 2017, accessed on June 19, 2018 .
- ↑ a b c GBL. In: drugscouts.de. Retrieved June 1, 2018 .
- ↑ a b c d e Fabian Dilger: The drug G! Retrieved January 8, 2020 .
- ↑ GHB, GBL & BDO (Liquid Ecstasy) | drugscouts.de. Retrieved June 1, 2018 .
- ^ A b Felix Betzler: GHB - Complications in withdrawal and their avoidance. Retrieved January 8, 2020 .
- ↑ "And then the lights go out". Retrieved January 8, 2020 .
- ↑ European Monitoring Center for Drugs and Drug Addiction (Ed.): European Drugs Report 2015: Trends and Developments . Publications Office of the European Union, Luxembourg 2015, ISBN 978-92-9168-799-2 , doi : 10.2810 / 91743 .
- ↑ Excerpt from the 2008 Fusion program booklet published on Flickr by taz author Julia Seeliger , published on July 1, 2008, accessed on October 1, 2015.
- ↑ John Lucas: A night in Berlin's most famous sex club - the Kit Kat . In: VICE . October 1, 2015, accessed October 1, 2015.
- ↑ a b "KO means" / "KO drops" . ( Memento from October 6, 2015 in the Internet Archive ) Forensic-Toxicological Laboratory Vienna, publication: unknown; accessed on October 5, 2015.
- ↑ A major raid against drug dealers. In: Spiegel online. July 9, 2008, accessed June 26, 2010 .
- ↑ D. Gee, P. Owen, L. Mclean et al. a .: Operation MATISSE: investigating drug facilitated sexual assault . Association of Chief Police Officers (ACPO), London 2006; Police claim many "drugged" date-rape victims simply drunk . In: Evening Standard. November 15, 2006, accessed October 8, 2015.
- ↑ Author der_andere_held in the blog Grossstadtsurvivor : GHB / GBL Safer Use Guide , published on April 18, 2008, accessed on September 29, 2015.
- ↑ Julia Seeliger: 2C-B and GBL . on: taz.de , January 3, 2012, accessed on September 29, 2015.
- ↑ Claus Peter Müller: The biggest knockout drop is alcohol . on: faz.net , February 19, 2013, accessed September 29, 2015.
- ↑ Brailsford AD, Bartlett C, Kicman AT, Cowan DA: Increases in Serum Growth Hormone Concentrations Associated With GHB Administration. In: NCBI, doi: 10.1093 / jat / bkw107. Journal of Analytical Toxicology, 41 (1): 54-59, January 2017, accessed June 3, 2020 .
- ↑ David J. Nutt, Leslie A. King, Lawrence D. Phillips: Drug harms in the UK: a multicriteria decision analysis . In: The Lancet . tape 376 , no. 9752 , November 6, 2010, p. 1558-1565 , doi : 10.1016 / S0140-6736 (10) 61462-6 , PMID 21036393 .
- ↑ Liquid Ecstasy . Drug Aid Hildesheim, accessed on September 29, 2015.
- ↑ GHB, GBL & BDO (Liquid Ecstasy). eve & rave Münster e. V. of August 31, 2015, accessed on September 29, 2015.
- ↑ Jörg Auf dem Hövel: Butandiol - GHB , published on December 24, 2002, accessed on September 29, 2015.
- ↑ GBL , SZL Suchtzentrum gGmbH, accessed on September 29, 2015.
- ↑ Carmen Pedraza, Francisca Belén García, José Francisco Navarro: Neurotoxic effects induced by gammahydroxybutyric acid (GHB) in male rats. ( Memento of September 29, 2015 in the Internet Archive ) March 17, 2009, accessed on September 29, 2015.
- ↑ Jennifer Hillebrand, Deborah Olszewski, Roumen Sedefov: GHB and its precursor GBL: an emerging trend case study. (PDF) 2008, accessed September 29, 2015.
- ^ Narcotics crime - Federal situation report 2014 . ( Memento of October 29, 2016 in the Internet Archive ) (PDF) Federal Criminal Police Office, published on December 8, 2015; Retrieved April 28, 2016.
- ↑ A drug experiment with wheel cleaner was planned . ( Memento from October 30, 2015 in the Internet Archive ) NDR, October 30, 2015; Retrieved April 28, 2016.
- ↑ DPA: Death by knockout drops - man sentenced to two and a half years in prison . on: Abendzeitung-muenchen.de , December 10, 2015, accessed on April 28, 2016.
- ↑ a b Verifiability . Drug Aid Cologne gGmbH, accessed on October 8, 2015.
- ↑ 8 Tips for Immediate Aid . ( Memento of November 7, 2015 in the Internet Archive ) LARA crisis and counseling center for raped and sexually harassed women, publication: unknown, accessed on October 6, 2015.
- ↑ Burkhard Madea, Frank Mußhoff: Knockout media: frequency, mode of action, Evidence assurance . In: Deutsches Ärzteblatt . tape 106 , no. 20 , 2009, p. 341–347 , doi : 10.3238 / arztebl.2009.0341 ( aerzteblatt.de ).
- ↑ Hair tests for the detection of narcotics and drugs (co-agents) . ( Memento of January 8, 2016 in the Internet Archive ) (PDF) Forensic-Toxicological Laboratory Vienna, July 1, 2013; accessed on October 5, 2015.