Olanzapine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Olanzapine | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 17 H 20 N 4 S | ||||||||||||||||||
| Brief description |
yellow, crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 312.43 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
195 ° C |
||||||||||||||||||
| pK s value |
4.69; 7.37 |
||||||||||||||||||
| solubility |
practically insoluble in water; Easily soluble in dilute mineral acids |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Olanzapine is an atypical neuroleptic drug that is mainly used in psychiatry to treat schizophrenic psychoses . It was introduced in Germany in 1996 under the name Zyprexa .
properties
The substance is closely related to clozapine in terms of its structure . In contrast to this, it can cause extrapyramidal motor disorders (EPS) - especially in high doses . However, the risk of agranulocytosis is lower with olanzapine than with clozapine. Overall, olanzapine triggers EPS much less often or to a lesser extent than older neuroleptics such as B. Haloperidol .
The compliance is often affected by a strong weight gain and drowsiness in olanzapine treatment. The cause of weight gain, which occurs more frequently with clozapine and olanzapine than with other neuroleptics, is suspected to be an interference with the action of insulin , so that the carbohydrate metabolism is impaired. This could also explain the increased risk of developing diabetes with olanzapine treatment .
Pharmacodynamics
Olanzapine blocks muscarinic acetylcholine receptors (mACH), serotonin receptors (5-HT 2 ) and dopamine receptors (D1 – D5). Alpha1 adrenoceptors and histamine 1 receptors are also inhibited. The half-life is 23–43 hours (extended in older patients), the bioavailability is 80%. Olanzapine is broken down in the liver .
Indications
Olanzapine is approved for the treatment of schizophrenia and for the therapy of bipolar disorders , provided that a manic phase has responded to the treatment. It is also used for obsessive-compulsive disorders . Olanzapine can also be used with an antidepressant to treat depression and anxiety disorders when treatment with an antidepressant alone has not been effective. Olanzapine also has a calming and relaxing effect that can reduce aggressive behavior.
Studies suggest that olanzapine can be used in severe and therapy-resistant forms of post-traumatic stress disorder . It seems to be helpful for nightmares and insomnia.
The drug also improves the effectiveness of antiemetic drugs during chemotherapy.
augmentation
According to a placebo-controlled study and clinical experience, the addition of olanzapine to unsuccessful antidepressant treatment (resistance to therapy ) may have a mood-enhancing effect. The addition of olanzapine has the advantage of being easy to carry out, but the points listed below must be observed with regard to interactions. The metabolism of antidepressants is not influenced by augmentation (combining several drugs to increase effectiveness).
Contraindications
In angle-closure glaucoma , psychotic states in elderly dementia patients and when a neuroleptic malignant syndrome (NMS) has preceded, olanzapine should not be used.
Side effects
Known side effects of olanzapine include the following:
- Weight gain
- sleepiness
- Circulatory disorders due to low blood pressure
- increased plasma prolactin level
- increased number of certain white blood cells
- Decrease in the number of white blood cells
- Decrease in the number of certain white blood cells
- increased cholesterol
- increased blood sugar level
- increased fat levels in the blood
- increased sugar levels in the urine
- Increase in appetite
- dizziness
- motor restlessness
- Parkinson's Disease Symptoms
- Movement disorders
- constipation
- Dry mouth
- Increase in liver enzymes, especially when starting treatment
- Rash
- Joint pain
- erectile dysfunction
- decreased libido
- general weakness
- fatigue
- Water retention
- fever
- Change in blood values
- immunological hypersensitivity
- Development or worsening of diabetes
- Seizures
- Eye cramps
- Memory problems
- Speech disorders
- Decreased heart rate
- Deviations in the EKG
- Thromboembolism
- Pulmonary embolism
- Venous thrombosis
- Epistaxis
- Flatulence
- Hypersensitivity to light
- Hair loss
- Bladder weakness
- Urinary retention
- Difficulty urinating
- Menstrual irregularities
- Breast enlargements
- Secretion of milk from the breast
- Breast formation in men
Ketoacidosis
A team of forensic scientists investigated the association of deaths from ketoacidotic (diabetic) metabolic imbalances in association with various antipsychotics and published that the most common drugs associated with these deaths were quetiapine and olanzapine, followed by risperidone. In 16 of 17 deaths examined, medication was defined as the primary or at least contributing factor in death from ketoacidosis. The risk of developing ketoacidosis increased with olanzapine in another study with the duration of treatment, while it appeared to cease with risperidone.
These and other reports led to the recommendation being made that blood glucose should be monitored regularly during therapy with atypical antipsychotics.
Intramuscular application
The FDA is currently investigating two deaths that occurred three and four days after intramuscular injection of olanzapine. Even if the cause of death has not yet been determined, increased serum concentrations of Zypadhera could be found, an increased release from the depot is considered likely.
The product information already points out the possibility of overdosing through accidental injection into the vicinity of blood vessels. There is a strict contraindication for intravenous use due to the severe complications. Overdosing can lead to PDSS (postinjection delirium sedation syndrome), cardiac arrhythmias and sudden cardiac arrest.
criticism
The company Eli Lilly, which was the first to market and patent olanzapine under the trade name Zyprexa, hit the headlines in 2006 ("Zyprexa scandal") because it was aware of certain side effects of the drug, such as drastic weight gain and possible manifestations had concealed a diabetes disease. Since 2005, a total of $ 1.2 billion in out-of-court compensation payments have been made in 28,500 cases (as of February 2007). In order to compensate for the declining revenues due to this scandal in the USA, the company increased the price of its product Zyprexa in Germany.
Interactions
As olanzapine is metabolised by CYP 1A2, caution should be exercised with substances that specifically induce or inhibit this isoenzyme. For example, fluvoxamine , a specific CYP 1A2 inhibitor , has been shown to significantly inhibit olanzapine metabolism. The mean increases in olanzapine AUC were 52% and 108%, respectively. A lower starting dose of olanzapine must be considered here. The same applies to taking it at the same time as ciprofloxacin .
Smoking and carbamazepine also affect olanzapine levels, which can lead to lower olanzapine concentrations.
The simultaneous administration of olanzapine and other medicinal products that prolong the QT interval in the ECG (e.g. ziprasidone ) should be avoided. The seizure threshold lowering drugs should not be used or only with great caution at the same time as olanzapine also favors epileptic seizures.
Trade names and dosage forms
The trade name of the original Olanzapine preparation is Zyprexa ® , manufactured and sold by Eli Lilly . In Germany, Austria and Switzerland are film-coated tablets and orodispersible tablets for oral application. A parenteral preparation as an intramuscular depot form (olanzapine pamoate monohydrate) is sold under the trade name ZypAdhera ® . A fast-acting intramuscular preparation is also marketed. Since the patent protection for Zyprexa ® in Germany was in great doubt from the end of 2007 to December 2008 due to a (non-final) judgment of the Federal Patent Court, numerous generics were available on the German market at that time. On December 17, 2008, however, the Federal Court of Justice ruled in favor of the original manufacturer Eli Lilly that the patent is valid. The decision of the Federal Court of Justice is final. Numerous generic manufacturers then announced that they would stop selling their olanzapine preparations for the time being. After the patent expired, generics have been available in Germany since October 1, 2011. Generics are also available in Austria.
Manufacturing
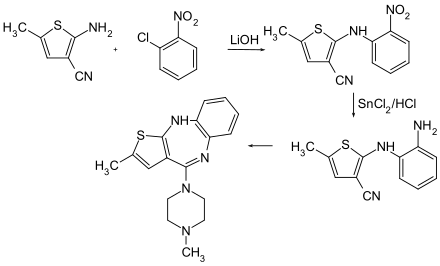
The multistage synthesis of olanzapine starts from 2-nitrochlorobenzene and 2-amino-3-cyano-5-methylthiophene, which are converted under the action of lithium hydroxide to the intermediate product 2- (2-nitroanilino) -5-methylthiophene-3-carbonitrile . The reduction of the nitro group is carried out with tin (II) chloride and hydrochloric acid . The cyclization to the seven-membered ring occurs immediately . Olanzapine is then formed by heating with N -methylpiperazine in a mixture of toluene and DMSO .
Trade names
Zypadhera (depot form in D, A), Zyprexa (D, A, CH), and numerous generics.
See also
Web links
- European Public Assessment Report (EPAR) and product information for Zalasta on the European Medicines Agency website
- rating
- Olanzapine . In: Erowid . (English)
Individual evidence
- ↑ a b c d Entry on olanzapine. In: Römpp Online . Georg Thieme Verlag, accessed on July 20, 2019.
- ↑ a b The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition, 2006, ISBN 978-0-911910-00-1 , p. 1175.
- ↑ a b Olanzapine data sheet from Sigma-Aldrich , accessed on April 8, 2019 ( PDF ).
- ↑ ePsy.de: Psychopharmaka Zeittafel .
- ↑ RM Navari: Olanzapine for the prevention and treatment of chronic nausea and chemotherapy-induced nausea and vomiting. In: European journal of pharmacology. Volume 722, January 2014, pp. 180-186, doi: 10.1016 / j.ejphar.2013.08.048 . PMID 24157985 .
- ^ O. Benkert, Hanns Hippius: Compendium of Psychiatric Pharmacotherapy . 12th, completely revised and updated edition. Berlin / Heidelberg 2019, ISBN 978-3-662-57334-1 .
- ↑ MM Söderberg, ML Dahl: Pharmacogenetics of olanzapine metabolism. In: Pharmacogenomics. Volume 14, Number 11, August 2013, pp. 1319-1336, doi: 10.2217 / pgs.13.120 . PMID 23930678 .
- ↑ Swiss Society for Obsessive Compulsive Disorders: Drug treatment of obsessive-compulsive disorder .
- ↑ M. Jakovljević, M. Sagud, A. Mihaljević-Peles: Olanzapine in the treatment-resistant, combat-related PTSD - a series of case reports. Acta Psychiatrica Scandinavica 26 (1): pp. 45-49, 2006, PMID 12752037 .
- ↑ rme / aerzteblatt.de: Chemotherapy: Olanzapine improves the effect of antiemetics. In: aerzteblatt.de . July 15, 2016. Retrieved July 19, 2016 .
- ↑ J. Schöpf: Modern antidepressants - changing, combining, augmenting . Steinkopff, Darmstadt 2003, ISBN 3-7985-1426-7 .
- ↑ OLANZAPIN-1A Pharma 5 mg film-coated tablets , pharmacy shop
- ↑ Susan F. Ely, Amber R. Neitzel, James R. Gill: Fatal Diabetic Ketoacidosis and Antipsychotic Medication . In: Journal of forensic sciences . December 27, 2012. doi : 10.1111 / 1556-4029.12044 .
- ↑ Krishnan Ramaswamy, Chris M Kozma, Henry Nasrallah: Risk of diabetic ketoacidosis after exposure to risperidone or olanzapine . In: Drug Safety . 30, No. 7, 2007, pp. 589-599.
- ↑ Melanie D. Guenette, Margaret Hahn, Tony A. Cohn, Celine Teo, Gary J. Remington: Atypical antipsychotics and diabetic ketoacidosis: a review . In: Psychopharmacology . 226, No. 1, March 2013, pp. 1-12. doi : 10.1007 / s00213-013-2982-3 .
- ↑ Cristian Palmiere, Daniel Bardy, Patrice Mangin, Dominique Werner: Postmortem diagnosis of unsuspected diabetes mellitus . In: Forensic Science International . August. doi : 10.1016 / j.forsciint.2013.01.004 .
- ↑ FDA Drug Safety Communication: FDA is investigating two deaths following injection of long-acting antipsychotic Zyprexa Relprevv (olanzapine pamoate)
- ↑ Ärzteblatt : USA: Lilly pays 500 million US dollars for side effects of Zyprexa® ( Memento of the original dated April 2, 2018 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , January 5, 2007.
- ^ Press release from Eli Lilly of December 18, 2008: Federal Court of Justice re-grants Lilly the Zyprexa patent .
- ^ A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances . 4th edition, 2 volumes published by Thieme-Verlag, Stuttgart 2000, ISBN 978-1-58890-031-9 ; online since 2003 with biannual additions and updates.