Haloperidol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
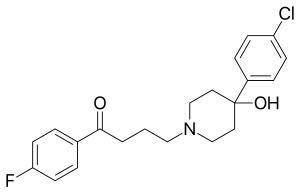
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Haloperidol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 21 H 23 ClFNO 2 | ||||||||||||||||||
| Brief description |
White to almost white powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 375,86 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
151.5 ° C |
||||||||||||||||||
| pK s value |
8.66 |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Haloperidol is a highly potent neuroleptic from the group of butyrophenones and is used, among other things, for the treatment of acute and chronic schizophrenic syndromes and for acute psychomotor states of excitement .
Development history
Haloperidol was on 11 February 1958 under Bert Hermans - an employee Paul Janssen in Beerse - in the search for a new opioid - analgesic synthesized as R1625 and in 1959 Belgium newly registered. Haloperidol quickly became the drug of choice for schizophrenia in Europe; it wasn't approved in the US until 1988. American psychiatrists preferred the highly potent phenothiazine preparation perphenazine .
Mode of action
Neuroleptics (also known as antipsychotics ) are often compared in their potency with chlorpromazine , the first antipsychotically active substance used in modern pharmacologically oriented psychiatry . Haloperidol has an antipsychotic effect about 50 times higher than its predecessor with reduced vegetative side effects, such as dry mouth and tachycardia , and in this regard is to be assessed as tolerated. However, this advantage is offset by the “motor” side effects typical of haloperidol.
The "higher antipsychotic effect" of haloperidol compared to chlorpromazine only relates to the required amount of the substance. The effectiveness of the substances at a comparable dosage is similar.
Haloperidol blocks dopamine receptors , especially subtype D 2 . The blockage of muscarinic and adrenergic receptors , which may cause undesirable effects, is less pronounced than with the previous antipsychotic.
As with all antipsychotics, there are two effects to be distinguished: an acute one and a long-term one. The primary effect is described by outsiders as well as by patients (in the context of their ability to express themselves possibly impaired by illness) as dampening and sedating , so this effect can be desired in pathologically relevant states of excitement. The actual antipsychotic effect only occurs when used for a few days to weeks. As a primary drug therapy, the substance can therefore help to eliminate undesirable symptoms such as those that occur in schizophrenia, but also in mania .
Haloperidol accumulates in the brain and other organs of the body about 20 times that of the blood. After stopping a haloperidol medication, its brain concentration drops only slowly. This explains the clinical observation that some side effects of haloperidol only slowly subside even after discontinuation.
application areas
In Germany, haloperidol is used to treat
- acute and chronic schizophrenic syndromes,
- organically conditioned psychoses ,
- acute manic syndromes and
- acute psychomotor agitation .
Haloperidol is usually used to suppress symptoms such as B. delusions , hallucinations or disorders of thought and consciousness as well as used to prevent relapses.
Furthermore, after all other treatment options have been exhausted, haloperidol can also be used to treat tic diseases (such as Gilles de la Tourette syndrome ). In Switzerland , haloperidol is also used to treat
- cerebral sclerotic restlessness,
- Oligophrenia with increased excitability,
- States of excitement in alcohol withdrawal syndrome ,
- Nausea and vomiting of various causes (if the usual drugs against nausea and vomiting are insufficiently effective) and
- as an accompanying medication for pain relief in various severe chronic pain conditions
authorized.
Like other neuroleptics of the phenothiazine type, haloperidol also works against nausea and vomiting, especially if psychological components play a role in the development of the symptoms.
Haloperidol was also used as a knockout drop by criminals.
Limitations 2017
The application was restricted in December 2017 for children and old people and for some areas of application. The occasion was an EU-wide harmonization of medicinal products containing haloperidol. Three indications were deleted or restricted due to a negative risk-benefit ratio or insufficient data. Children under the age of 10 should no longer be treated with haloperidol. The maximum dose for adults is 10 to 20 mg daily, regardless of the indication, 5 mg for elderly patients and 3 to 5 mg daily for children. A depot application is only permitted if the patient has previously been stably adjusted to oral haloperidol.
Side effects
- Tardive dyskinesia (swallowing and throat cramps, "lumpy" language, dystonic movements)
- Signs of fatigue
- Agitation
- Sitting restlessness ( akathisia )
- Extrapyramidal Syndrome
- Hypotension (especially if there is a lack of volume)
- Orthostatic dysregulations
- Conduction disorders ( QTc time prolongation , AV block , bundle branch block )
- Paradoxical hypotension after adrenaline injection
- Speech disorders
- Hunger and weight gain
- Psychotic disorders , depression
While the vegetative side effects tend to take a back seat, the main side effects of haloperidol are an influence on the extrapyramidal motor function . These symptoms, which are reminiscent of Parkinson's disease , are called Parkinsonoid and, according to current observations, are largely reversible and also dose-dependent after the end of the administration of the substance. Visible symptoms include abnormal movements in the head and neck, and difficulty speaking and swallowing. During administration, such side effects are often treated by co-medication with the anti- Parkinsonian drug Biperiden . A complete regression of the side effects is not to be expected in every case.
Haloperidol can severely limit the ability to experience and emotionality and thus lead to a "mental flattening". This is probably the reason for the frequent lack of compliance . It is discussed that haloperidol should therefore not be used e.g. B. in schizophrenia as a permanent prophylactic, but should only be given acutely until the symptoms subside; then a long-term treatment with atypical, more modern neuroleptics should be aimed for.
Interactions
Haloperidol can be weakened in its effect by other drugs such as phenytoin , as well as reducing the effect of other drugs ( bromocriptine , levodopa , phenylephrine ) or increasing desired or undesired effects (central nervous effects of methyldopa , respiratory depressive effects of certain antibiotics).
reception
In some cases, haloperidol has been referred to as a “ concrete syringe ” for immobilizing patients in psychiatry and in prison . This term refers to the typical motor restrictions (gait) associated with haloperidol medication. The administration of haloperidol against the patient's will was described as one of the neglected news items of 2016 by the News Awareness Initiative .
Dosage forms
Haloperidol is available in various dosage forms for oral intake ( tablets and drops) and as an injection solution for intramuscular injection, here also as a depot form. Intravenous administration is no longer recommended due to possible cardiac side effects.
Trade names
Monopreparations : Haldol (D, A, CH), Serenase (Italy), various generics (D)
See also
literature
- Franz Strehle: Studies on the mechanism of action of carbamazepine and haloperidol. Dissertation . University of Munich , 1992.
Web links
- Questions and answers about neuroleptics
- Haloperidol. ( Memento from February 11, 2013 in the web archive archive.today ) In: Psychiatrie-aktuell.de
Individual evidence
- ↑ a b European Pharmacopoeia Commission (ed.): EUROPEAN PHARMACOPOE 5TH EDITION . tape 5.0-5.8 , 2006.
- ↑ a b c d Entry on haloperidol in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ K. Takács-Novák, M. Urac, P. Horváth, G. Völgyi, BD Anderson, A. Avdeef: Equilibrium solubility measurement of compounds with low dissolution rate by Higuchi's Facilitated Dissolution Method. A validation study. In: Eur. J. Pharm. Sci. 106, 2017, pp. 133-144, doi: 10.1016 / j.ejps.2017.05.064 .
- ↑ a b Data sheet Haloperidol from Sigma-Aldrich , accessed on April 3, 2011 ( PDF ).
- ↑ a b c Haldol-Janssen, solution: Haloperidol. ( Memento of the original from May 19, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. accessed on January 13, 2016.
- ↑ B. Granger: The discovery of haloperidol. In: Encephale. 25 (1), Jan-Feb 1999, pp. 59-66. PMID 10205735 . (Article in French, English abstract).
- ^ B. Granger, S. Albu: The haloperidol story. In: Ann Clin Psychiatry. 17, 2005, pp. 137-140. PMID 16433054 .
- ↑ ePsy.de: Haloperidol
- ↑ a b J. Kornhuber, A. Schultz, J. Wiltfang, I. Meineke, CH Gleiter, R. Zöchling, KW Boissl, F. Leblhuber, P. Riederer: Persistence of haloperidol in human brain tissue. In: Am.J. Psychiatry. 156, 1999, pp. 885-890. PMID 10360127
- ↑ J. Kornhuber, J. Wiltfang, P. Riederer, S. Bleich: Neuroleptic drugs in the human brain: clinical impact of persistence and region-specific distribution. In: Eur.Arch.Psychiatry Clin.Neurosci. 256, 2006, pp. 274-280. PMID 16788768
- ^ CM Eddy, HE Rickards, AE Cavanna: Treatment strategies for tics in Tourette syndrome. In: Therapeutic advances in neurological disorders. Volume 4, number 1, January 2011, pp. 25-45, doi: 10.1177 / 1756285610390261 . PMID 21339906 , PMC 3036957 (free full text).
- ↑ Janssen-Cilag: Haldol. Specialist information from the Swiss Medicines Compendium. As of May 2008.
- ↑ Eberhard Aulbert, Wiebke Nehls: Palliative internistic-oncological tumor therapy. In: Eberhard Aulbert, Friedemann Nauck, Lukas Radbruch (eds.): Textbook of palliative medicine. With a foreword by Heinz Pichlmaier. 3rd, updated edition. Schattauer, Stuttgart 2012, ISBN 978-3-7945-2666-6 , pp. 633-663, here: p. 654.
- ↑ drogenkult.net
- ↑ Taverns: Half-gang types . In: Der Spiegel . No. 6 , 1985, pp. 205-206 ( online ).
- ↑ Haloperidol: Fewer Indications Approved - News from Adhoc Pharmacy
- ^ Tilman Wetterling: Psychiatric emergencies. In: Jörg Braun, Roland Preuss (Ed.): Clinic Guide Intensive Care Medicine. 9th edition. Elsevier, Munich 2016, ISBN 978-3-437-23763-8 , pp. 357-369, here: p. 365 f. ( Haloperidol ).
- ↑ 2016: Top 8 - "Concrete spraying" in psychiatry. In: Initiative news clearance . Retrieved September 29, 2019 (German).
- ↑ Drug Safety Mail 2010-098 of 5.5.2010. In: www.akdae.de. May 5, 2010, accessed March 30, 2016 .