Heptanal
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Heptanal | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 14 O | |||||||||||||||
| Brief description |
colorless liquid with an unpleasant odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 114.19 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.82 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−43 ° C |
|||||||||||||||
| boiling point |
153 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
poor in water (1.25 g l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.4113 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Heptanal is an aldehyde and a colorless liquid with a sharp unpleasant odor. The common name is heptaldehyde.
Occurrence and representation
Heptanal occurs naturally in the essential oils of ylang-ylang ( Cananga odorata ), clary sage ( Salvia sclarea ), lemon ( Citrus x limon ), bitter orange ( Citrus x aurantium ), rose ( Rosa ) and hyacinth ( Hyacinthus ). The formation of heptanal in the fractional distillation of castor oil was described as early as 1878. The industrial presentation is based on the pyrolytic cleavage of ricinoleic acid esters ( Arkema process) and on the hydroformylation of 1-hexene with rhodium -2-ethylhexanoate as a catalyst with the addition of 2-ethylhexanoic acid ( Oxea process):
properties
Heptanal is an inflammable, non-volatile, colorless liquid with a penetrating fruity to oily-fatty odor, which is miscible with alcohols and practically insoluble in water. Because of its sensitivity to oxidation, heptanal is bottled under nitrogen and stabilized with 100ppm hydroquinone .
Heptanal forms flammable vapor-air mixtures. The compound has a flash point of 39.5 ° C. The explosion range is between 1.1 vol.% As the lower explosion limit (LEL) and 5.2 vol.% As the upper explosion limit (UEL). The ignition temperature is 205 ° C. The substance therefore falls into temperature class T3.
use
1-heptanol can be produced from heptanal by reduction using hydrogen addition :
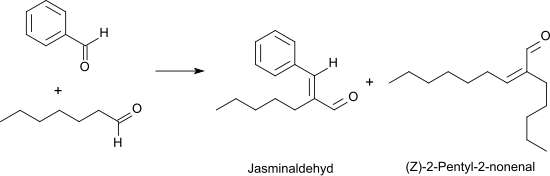
In the oxidation of heptanal with oxygen in the presence of rhodium catalysts at 50 ° C., heptanoic acid is obtained in 95% yield . In a Knoevenagel reaction , heptanal reacts with benzaldehyde under basic catalysis with high yield and selectivity (> 90%) to form alpha-pentylcinnamaldehyde (also called jasmine aldehyde because of its typical jasmine smell), which is mostly used as a cis / trans isomer mixture in perfume preparations.
The unpleasantly rancid smelling ( Z ) -2-pentyl-2-nonenal is produced as a by-product through self-condensation of the heptanal . In the presence of aqueous boric acid, heptanal can be converted virtually quantitatively into ( Z ) -2-pentyl-2-nonenal with azeotropic removal of the water .
Complete hydrogenation gives the branched primary alcohol 2-pentylnonan-1-ol , which can also be obtained from 1-heptanol by the Guerbet reaction .
safety instructions
Inhalation, ingestion or absorption through the skin can be harmful to health. It can irritate the respiratory tract, digestive tract and eyes: e.g. B. Burning, scratching. It can also irritate the skin: B. Burning, itching.
Individual evidence
- ↑ a b c d e f g h i j k l m n Entry on oenanthaldehyde in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-274.
- ↑ a b G. A. Burdock, Fenaroli's Handbook of Flavor Ingredients, Fifth Edition , 2005, CRC Press, Boca Raton, Fl., ISBN 0-8493-3034-3 .
- ^ F. Krafft, Distillation of castor oil, under educed pressure , Analyst, 3 , 329a (1878).
- ^ A. Chauvel, G. Lefebvre, Petrochemical Processes: Technical and Economic Characteristics , Volume 2, p. 277, Editions Technip, Paris, 1989, ISBN 2-7108-0563-4 .
- ↑ German patent DE 102007053385, process for the production of aldehydes , inventor: A. Fischbach et al., Applicant: Oxea Deutschland GmbH, published on May 20, 2009.
- ↑ Guide values for saturated acyclic aliphatic C4 to C11 aldehydes in indoor air, Bundesgesundheitsbl - Gesundheitsforsch –Gesundheitsschutz, 2009, 52: 650–659, doi : 10.1007 / s00103-009-0860-2 .
- ↑ Acros Organics, safety data sheet, heptaldehyde, stabilized , revised. on November 19, 2012.
- ↑ German patent DE 10010771, process for the production of aliphatic carboxylic acids from aldehydes , inventors: H. Springer, P. Lappe, applicant: Celanese Chem Europe GmbH, published on May 3, 2001.
- ↑ M. Perez-Sanchez, P. Dominguez de Maria, Synthesis of natural fragrance jasminaldehyde using silica-immobilized piperazine as organocatalyst , Catal. Sci. Technol., 3, 2732-2736 (2013), doi : 10.1039 / C3CY00313B
- ↑ Fragrance Lexicon A, alpha-amylcinnamaldehyde , last change on August 4, 2000.
- ↑ JM Hornback, Organic Chemistry, 2nd edition , p. 886, Thomson Brooks / Cole, 2006, ISBN 0-534-49317-3 .
- ↑ RD Offenhauer, SF Nelsen, Aldehyde and ketone condensation reactions catalyzed by boric acid , J. Org. Chem., 33 (2), 775-777 (1968), doi : 10.1012 / jo01266a059 (currently unavailable) .
- ^ GH Knothe: Lipid Chemistry, Guerbet Compounds , AOCS Lipid Library, December 22, 2011.