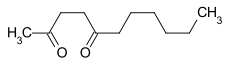
2,5 undecandione
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,5 undecandione | ||||||||||||||||||
| other names |
Undecane-2,5-dione |
||||||||||||||||||
| Molecular formula | C 11 H 20 O 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 184.27 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
0.901 g cm −3 |
||||||||||||||||||
| Melting point |
33 ° C |
||||||||||||||||||
| boiling point |
141 ° C (19 hPa) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2,5-Undecanedione is a chemical compound from the group of diketones in which the two carbonyl groups are separated by two methylene groups (γ-diketone).
presentation
According to a synthetic route described by Heinz Hunsdiecker in 1942, 2,5-undecanedione can be obtained starting from 5-methylfurfurol . First, 5-methylfurfural 1 is reacted with methyl propyl ketone 2 in an aldol condensation to form the α, β-unsaturated carbonyl compound 3 . With sodium amalgam , only the double bond of the side chain of the condensation product is specifically reduced . The intermediate stage 4 is then converted into 2-methyl-5-hexylfuran 5 in a Wolff-Kishner reduction with hydrazine . The furan ring is cleaved by acid-catalyzed conversion at 120 ° C. and converted to 2,5-undecanedione 6 .
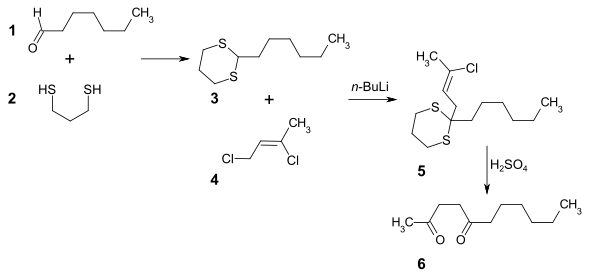
An alternative synthesis route starts from heptanal 1 . First, the carbonyl group is protected with 1,3-propanedithiol 2 as dithiane 3 . The dithiane is deprotonated in THF with n- butyllithium and alkylated with 1,3-dichloro-2-butene 4 to form intermediate 5 . The 2,5-undecanedione 6 is obtained by hydrolysis of the dithioacetal protective group with concentrated sulfuric acid . The crude product can be worked up and purified by derivatization with Girard reagent T in methanol.
use
2,5-undecanedione can be cyclized in a Hunsdiecker condensation with sodium hydroxide solution in boiling ethanol to the fragrance dihydrojasmone :
Individual evidence
- ↑ a b c Entry on Undecane-2,5-dione at ChemicalBook , accessed on March 17, 2020.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Heinz Hunsdiecker: About the behavior of γ-diketones, 1st communication . In: Reports of the German Chemical Society . tape 75 , no. 5 , May 6, 1942, pp. 447-454 , doi : 10.1002 / cber.19420750502 .
- ↑ External identifiers from or database links to Girard reagent T : CAS number: 123-46-6, EC number: 204-629-3, ECHA InfoCard: 100.004.210 , PubChem : 67156 , ChemSpider : 60501 , Wikidata : Q27285753 .
- ↑ Tse-Lok Ho, Honor C. Ho, CM Wong: A Synthetic Route to Dihydrojasmone; Sulfuric Acid as Dethioacetalization Agent . In: Canadian Journal of Chemistry . 51 (2), 1973, pp. 153-155, doi : 10.1139 / v73-023 .