Phylloquinone
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|
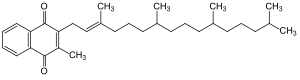
|
|||||||||
| General | |||||||||
| Common name | Vitamin K 1 | ||||||||
| other names |
|
||||||||
| Molecular formula | C 31 H 46 O 2 | ||||||||
| CAS number | 84-80-0 | ||||||||
| ATC code | |||||||||
| Brief description | yellow to green, clear, viscous liquid | ||||||||
| Occurrence | Cabbage , spinach , Brussels sprouts , cabbage sprouts | ||||||||
| physiology | |||||||||
| function | Blood clotting, bone metabolism, photosynthesis, vitamin K cycle | ||||||||
| Daily need | about 80 µg | ||||||||
| Consequences in case of deficiency | Slowing of blood clotting, cerebral hemorrhage in infants, indigestion | ||||||||
| Overdose | not known | ||||||||
| properties | |||||||||
| Molar mass | 450.71 g · mol -1 | ||||||||
| Physical state | liquid | ||||||||
| density | 0.984 g cm −3 (20 ° C) | ||||||||
| Melting point |
−20 ° C |
||||||||
| solubility | fat-soluble, also soluble in alcohol, benzene, chloroform, ether | ||||||||
| safety instructions | |||||||||
|
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Phylloquinone or vitamin K 1 is one of the fat-soluble K vitamins . In the human organism it does in areas such as blood clotting an important role.
It occurs naturally in the chloroplasts of green plants . As a normal component of the photosynthetic apparatus and partly in fruits, it occurs in different concentrations. In the photosynthesis of plants, it is involved in the electron transfer chain as cofactor A1.
The molecule consists of a methylnaphthoquinone with a phytyl side chain . At room temperature it is a viscous liquid.
history
In 1929 , vitamin K was isolated from alfalfa . In the year 1943 received Henrik Dam - for the discovery - and Edward Adelbert Doisy - for the discovery of the chemical nature of vitamin K - shared the Nobel Prize for Medicine . The artificial synthesis of vitamin K 1 was first achieved by Louis Frederick Fieser in 1939.
Names
Vitamin K 1 is the trivial name for 2-methyl-3-phytyl-1,4-naphthoquinone (also α-phylloquinone). The origins of the name phylloquinone from the word Phyllos, the sheet. The letter K was used after the Danish researcher Henrik Dam isolated a fat-soluble substance from dried alfalfa leaves around 1935 , which had a balancing effect on blood clotting (coagulation vitamin) and was then called vitamin K for the sake of simplicity . It's a terpenoid .
description
Vitamin K can only be absorbed with the help of bile acid . The absorption is increased with the simultaneous intake of fats. Vitamin K is involved in the production of various blood clotting factors in the liver. Vitamin K is also directly involved in building bones through the body's own proteins, for example osteocalcin, and through functions within calcium metabolism. Due to the heat stability of the vitamin K group, there is little vitamin loss during preparation, especially during cooking. Vitamin K is also stable to oxygen . When exposed to light, vitamin K becomes inactive and quickly loses its bioavailability.
All substances with phylloquinone activity (K vitamins) are derived from the naturally occurring 2-methyl-1,4-naphthoquinone (menadione). In addition to the unsubstituted, aromatic ring, a lipophilic side chain in the trans configuration is a prerequisite for vitamin K activity . Natural terpene chains with 20 carbon atoms are optimal . Side chains below eight carbon atoms lead to inactivity, except for menadione.
Up to 100 compounds with vitamin K activity are known, but only three of them are important.
Task / function
Blood clotting
The main function of vitamin K is that it is necessary for the synthesis of certain proteins ( prothrombin ), which play an important role in blood clotting. These factors stop bleeding (clotting). Vitamin K also plays a major role in the biosynthesis of proteins in bones, kidneys, plasma and connective tissue. As a fat-soluble vitamin, vitamin K 1 is bound to the absorption of fats. The resorption rate is 60–80 percent. Vitamin K 2 , on the other hand, reaches the intestinal tissue by diffusion. Vitamins K 1 and K 2 reach the bone marrow, liver and kidneys via the blood. Storage can take place here for up to 14 days. The vitamins are excreted through the bile and partly through the kidneys.
Vitamin K's biological activity is due to its ability to switch between its oxidized ( quinone ) and reduced ( hydroquinone ) forms in the vitamin K cycle. The essential importance of vitamin K lies in its contribution to the post-translational introduction of a carboxy group in the γ-position of glutamyl residues of specific proteins, which changes their properties. Its main function is to participate in the synthesis of various blood coagulation factors (factor X, IX, VII, II).
photosynthesis
Phylloquinone is contained in the photosynthesis reaction center Photosystem I, where it is involved in the electron transfer chain as a secondary electron acceptor (cofactor A1).
Vitamin K cycle
The peroxide of vitamin K changes to the epoxide of vitamin K and leads to deprotonation of the protein (e.g. the coagulation factor ). The protein is converted from ATP into γ-carboxylated protein (or vitamin K-dependent coagulation factor) by means of CO 2 and energy .
The epoxide of vitamin K is converted to the quinone of vitamin K by means of vitamin K epoxide reductase . The quinone of vitamin K is converted to the hydroquinone of vitamin K by means of carbonyl reductase . These two steps can be inhibited by the heart attack drugs dicoumarol , marcumar and warfarin .
The hydroquinone of vitamin K becomes the peroxide of vitamin K. The cycle starts all over again.
Occurrence
Vitamin K 1 is found in the lamellar membranes of the chloroplasts in green plants, while the vitamin K 2 forms are synthesized by the intestinal flora, among other things. The aromatic cycle of the K vitamins is formed in the so-called shikimic acid pathway . In the body, vitamin K is present in the blood plasma and stored in the liver, kidneys and spleen. It occurs in green vegetables (Brussels sprouts, kale, green tomatoes, spinach, broccoli, carrot greens, ...) and potatoes, rose hips, lettuce, soybeans, green tea, in milk and dairy products as well as lean meat. The content fluctuates, especially in plant-based foods, depending on the season and location.
An average human daily dose of 65 µg is contained in:
| Food | amount |
|---|---|
| chives | 15 g |
| Brussels sprouts | 25 g |
| Calf liver | 50 g |
| Eggs | 3 pieces |
| Quark | 220 g |
| Mushrooms | 400 g |
| Strawberries | 500 g |
Typical amounts of vitamin K 1 in food:
| Amount [g] | Food | Amount of vitamin K 1 [µg] |
|---|---|---|
| 100 | Kale | 729 |
| 100 | Brussels sprouts | 236 |
| 100 | cauliflower | 57 |
| 100 | Kohlrabi | 7th |
| 200 | Whole grain bread | 3.4 |
requirement
Determining the vitamin K requirement is difficult due to analytical problems in determining this vitamin in food and the uncertainty about the level of synthesis by bacteria in the intestine. With regard to the daily requirement for vitamin K there is a different assessment. The German Nutrition Society recommends: 65 µg for women and 80 µg for men per day. Since infants often suffer from a vitamin K deficiency because breast milk has only a low vitamin K content, vitamin K prophylaxis is often recommended.
Deficiency symptoms (hypovitaminosis)
With the exception of newborns , vitamin K deficiency in humans is rather rare, especially since it is a fat-soluble vitamin and the body's storage reserves are not exhausted as quickly as in the case of water-soluble vitamins. Liver and chronic stomach and intestinal diseases ( diarrhea ) promote a vitamin K deficiency; excessive alcohol consumption can be a reason for this. Oral intake of antibiotics (growth inhibition of the intestinal bacteria that supply vitamin K) can, however, inhibit the body's own vitamin K formation; but this only happens with simultaneous malnutrition. Furthermore, osteoporosis , where an increased loss of calcium is typical, often leads to a vitamin K deficiency. As with other fat-soluble vitamins, an intestinal fat absorption disorder ( e.g. biliary obstruction or exocrine pancreatic insufficiency ) can lead to deficiency symptoms. However, if there is a lack of vitamin K, blood coagulation slows down. Cerebral haemorrhage can occur in infants. Digestive disorders, chronic liver diseases and bleeding in various tissues and organs, such as on the nasal mucosa, in the gastrointestinal tract and in the muscles, are possible.
Consequences of an overdose (hypervitaminosis)
Since vitamin K has no toxic effects (no toxic effects are known for 500 times the recommended amount), there are hardly any negative effects from overdosing. After injecting vitamin K in very high doses, allergic reactions and changes in the composition of the blood can occur. Some people have a genetic defect in which an overdose of vitamin K can lead to thrombosis . A genetic test for this is available.
Antagonists
It is known that vitamin K is essential for the synthesis of coagulation factors (prothrombin). The presence of vitamin K antagonists (e.g. warfarin , dicumarol) clarified the mode of action of this vitamin for the first time. The dicumarol in spoiled clover, for example, led to life-threatening bleeding in cattle. Warfarin is also used as a rat poison . Cows fed dicumarol have abnormal prothrombin which, unlike normal prothrombin, no longer binds Ca 2+ . This comes about by changing an amino acid in prothrombin.
As a drug to inhibit blood clotting, phenprocoumon is often used as an antagonist.
Trade names
KA-Vit (D), Konakion (D, A, CH)
FrekaVit (D), Vitalipid (D, A, CH)
Web links
- vitamin-k1.de: A forgotten vitamin gets new acceptance ( Memento from January 30, 2009 in the Internet Archive )
- Vitamin K content of foods
literature
- O. Isler: About vitamins K 1 and K 2 . In: Angewandte Chemie . tape 71 , no. 1 , 1959, p. 7-15 , doi : 10.1002 / anie.19590710103 .
Individual evidence
- ↑ a b c d e data sheet Phylloquinone (K1), analytical standard at Sigma-Aldrich , accessed on June 25, 2017 ( PDF ).
- ↑ MJ Shearer: Vitamin K. In: Lancet. Volume 345, Number 8944, January 1995, pp. 229-234, PMID 7823718 (Review).
- ↑ RT Weibert, DT Le, SR Kayser, SI Rapaport: Correction of excessive anticoagulation with low-dose oral vitamin K1. In: Annals of internal medicine. Volume 126, Number 12, June 1997, pp. 959-962, PMID 9182473 .
- ↑ Secondary Hemostasis - Part 3 - Coagulation on Negative Surfaces - Vitamin K on YouTube , accessed June 3, 2016.
- ↑ F. MacMillan, J. Hanley, L. van der Weerd, M. Knüpling, S. Un, AW Rutherford: Orientation of the phylloquinone electron acceptor anion radical in photosystem I. In: Biochemistry. Volume 36, Number 31, August 1997, pp. 9297-9303, doi : 10.1021 / bi971097d , PMID 9280439 .
- ↑ Markus Linnemann, Michael Kühl, T. Holletz, S. Güler: Biochemistry for medical professionals : A learning and work book with clinical relevance. 7th edition, Springer-Verlag, 2006, ISBN 978-3-540-21176-1 , p. 772.


