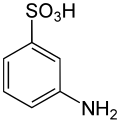
Metanilic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Metanilic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 7 NO 3 S | ||||||||||||||||||
| Brief description |
white, crystalline solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 173.19 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.69 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
288 ° C (decomposition) |
||||||||||||||||||
| solubility |
little in water (12.8 g l −1 at 7 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Metanilic acid is the common name for 3-aminobenzenesulfonic acid . Aminobenzenesulfonic acids are important industrial intermediates for the synthesis of organic , water-soluble dyes . Due to the high acidity of the sulfonic acid function, in contrast to aminocarboxylic acids , their isoelectric point (IEP) is acidic.
synthesis
Industrial production starts from nitrobenzene , which is first selectively sulfonated to 3-nitrobenzenesulfonic acid . This is followed by a Béchamp reduction .
Alternatively, benzenesulfonic acid can also be nitrated , whereby an isomer mixture of nitrobenzenesulfonic acids is formed, which has to be separated. The meta-isomer is then reduced to metanilic acid as above. This process is used when orthanilic acid is to be obtained at the same time .
application
Metanilic acid is used as a reagent in the detection of nitrites .
Individual evidence
- ↑ a b c d e f Entry on 3-aminobenzenesulphonic acid in the GESTIS substance database of the IFA , accessed on February 14, 2017(JavaScript required) .
- ↑ Entry on 3-aminobenzenesulphonic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
