Aniline yellow
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
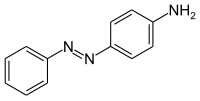
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Aniline yellow | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 12 H 11 N 3 | ||||||||||||||||||
| Brief description |
yellow leaflets or needles |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 197.24 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.19 g cm −3 |
||||||||||||||||||
| Melting point |
121-124 ° C |
||||||||||||||||||
| boiling point |
> 360 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Authorization procedure under REACH |
particularly worrying : carcinogenic ( CMR ) |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Aniline yellow (p-aminoazobenzene) is a yellow azo dye . It is a derivative of azobenzene and at the same time an aromatic amine .
history
Aniline yellow was the first synthetic azo dye.
In 1860, the German chemist Peter Grieß described the synthesis of an explosive substance crystallizing in golden yellow platelets, which he had obtained from the action of nitrous acid on an alcoholic aniline solution, before C. Mene and Hugo Schiff described the production of a yellow dye in the following year in the first case again through the action of nitrous acid on aniline , in the second case, on the other hand, through the action of sodium stannate or sodium antimonate as oxidizing agents on aniline.
Simpson, Maule and Nicholson, who put this dye on the market in 1864 as the second azo dye (after the aniline black discovered in 1863 and brought onto the market in the same year ), initially assumed that the aniline yellow produced by C. Mene's process and the substance described by Grieß are identical. Why they completely ignored the explosive properties of the latter is unknown.
The discrepancy gave rise to further investigations by Grieß himself and Carl Alexander von Martius , who showed that the "diazoamidobenzene" produced by Grieß in 1860 decomposes in boiling hydrochloric acid with the elimination of nitrogen into phenol and aniline, while the oxalate of "amidodiphenylimide" is commercially available Aniline yellow located under the same conditions neither exhibits nitrogen evolution nor phenol and aniline as decomposition products. In addition, the aniline yellow produced by Mene's process had a clearly basic reaction, while Grieß's "diazoamidobenzene" showed hardly any basic behavior.
Since the ring-shaped structure of benzene was still largely unknown at this point in time, they were unable to provide any more detailed information about the exact structure of the two compounds, but at least recognized their isomeric character based on their same elemental composition and the fact that both belong to the same class as the found azobenzene discovered by Mitscherlich .
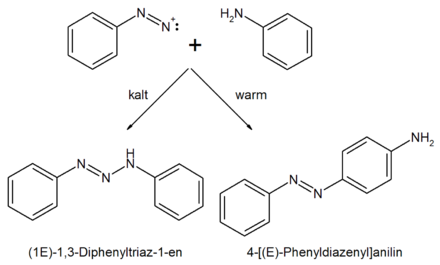
The most important result of these investigations, however, was the finding that the formation of one or the other product was dependent on the temperature: While the formation of the explosive "diazoamidobenzene" predominated in the cold, the addition of heat led to the formation of the "amidodiphenylimide" or Aniline pounds.
From today's perspective, the results can be interpreted as follows:
After the aniline is diazotized as known (see diazotization ), the resulting diazonium cation reacts in the warmth with another aniline molecule to form the thermodynamically more stable p- aminoazobenzene (IUPAC: 4 - [( E ) -phenyldiazenyl] aniline, thermodynamic control), at low temperature on the other hand, with the nitrogen atom of the amino group of the aniline as its most nucleophilic position with formation of a triazene ( IUPAC : (1 E ) -1,3-diphenyltriaz-1-ene, kinetic product control).
Extraction and presentation
Aniline yellow can be obtained by the action of nitrous acid on aniline and by acid rearrangement of 1,3-diphenyl triazene .
properties
Aniline yellow crystallizes in yellow needles or leaflets, amorphous aniline yellow is an orange powder that is highly carcinogenic and is also suspected of being mutagenic .
proof
Brief description: Reduction of the sample with dithionite , extraction of the aromatic amines with MTBE and chromatographic detection of the amines ( TLC , HPLC -DAD / MSD).
use
Aniline yellow was used, among other things, as a staining agent in microscopy .
Legal status
Aniline yellow is included as a carcinogenic amine in the list of aromatic amines for which legal limit values have been set in the EU (2008: 30 ppm ). The import and sale of consumer goods that exceed the specified limit values is prohibited in the EU. The implementation in national law takes place via the Consumer Goods Ordinance . The determination takes place according to the analytical method in the official collection of examination procedures § 35 of the LMBG under the division number B − 82.02 .
literature
- Entry for 4-aminoazobenzene. In: Römpp Online . Georg Thieme Verlag, accessed on January 2, 2015.
Individual evidence
- ↑ a b c d e f Entry on 4-aminoazobenzene in the GESTIS substance database of the IFA , accessed on February 22, 2017(JavaScript required) .
- ↑ Handbook of Chemistry and Physics , 70th edition, 1989–1990.
- ↑ Entry on 4-aminoazobenzene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Anilingelb data sheet from Sigma-Aldrich , accessed on November 5, 2016 ( PDF ).
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on July 17, 2014.
- ↑ Entry on aniline yellow in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Peter Griess: Preliminary note on some new compounds that contain hydrogen represented by nitrogen. In: Annals of Chemistry and Pharmacy. 113, 1860, p. 334, doi : 10.1002 / jlac.18601130307 .
- ↑ C. Mene: Wagner's Annual Reports 7, 1861. P. 496.
- ↑ H. Schiff: Further studies on aniline dyes. In: Annals of Chemistry and Pharmacy. 127, 1863, p. 342, doi : 10.1002 / jlac.18631270310 .
- ↑ CA Martius, P. Griess: About the amidodiphenylimide, a new organic base. In: Journal for Practical Chemistry. 97, 1866, p. 257, doi : 10.1002 / prac.18660970134 .
- ^ FA Kekulé: Zeitschrift für Chemie 6 (1865); P. 176.
- ^ E. Mitscherlich: Annalen der Physik und Chemie XXXII (1834). P. 224.
- ↑ E. Mitscherlich: About nitrogen benzide. In: Annals of Pharmacy. 12, 1834, p. 311, doi : 10.1002 / jlac.18340120282 .
- ^ RL Howey: Vital Staining for Protozoa and Related Temporary Mounting Techniques . 2000.