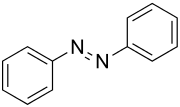
Azobenzene
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| Structural formula of ( E ) -azobenzene | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Azobenzene | ||||||||||||||||||
| other names |
Diphenyldiazene ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 12 H 10 N 2 | ||||||||||||||||||
| Brief description |
orange-red leaflets |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 182.22 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.203 g cm −3 |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| boiling point |
293 ° C [( E ) -azobenzene, undecomposed] |
||||||||||||||||||
| solubility | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Azobenzene is the simplest aromatic azo compound . It consists of two phenyl groups linked by an azo bridge (–N = N–). It is the parent substance of numerous azo dyes .
history
Six years after the start of the generally accepted "organic synthesis" ( F. Wohler , urea from ammonium cyanate ) and 22 years before WH Perkin ( Mauvein ) reported E. Mitscherlich a red compound, which he distillation of nitrobenzene with potassium hydroxide solution obtained. He called it azobenzene.
For a long time nothing was known about the constitution of this connection. Mitscherlich himself suggested C 12 H 5 N as the empirical formula . On the basis of steam density measurements , other authors came up with the empirical formula C 24 H 10 N 2 . It was not until 1860 that the correct sum formula was postulated. FA Kekulé finally made the first correct structural proposal in 1866.
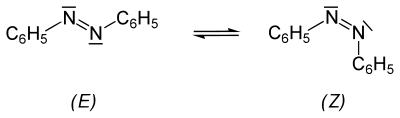
The question of the configuration of the N = N double bond remained unresolved . For analogous compounds ( diazo hydroxides ), A. Hantzsch proposed isomerism in 1921 , then called "syn / anti isomerism". The IUPAC now recommends the use of ( E , Z ) nomenclature.
Isomers
In 1937, S. Hartley found a second, yellow modification by exposing azobenzene to light . He was able to separate the yellow isomer by chromatography . The exact configuration of the two isomers was proven by an X-ray structure analysis in 1939 . Azobenzene therefore exists in the form of two isomers (see cis - trans isomerism ), which differ in color, solubility, chromatographic behavior, etc.
When a solution of ( E ) -azobenzene is irradiated with UV light , this partially changes into the ( Z ) form in an equilibrium reaction ; depending on the solvent , 15-40% ( Z ) -azobenzene is formed. Pure ( Z ) -azobenzene slowly converts thermally in the solid or in the melt into the more stable ( E ) -isomer. The enthalpy of reaction of isomerization in the melt is −48.2 kJ mol −1 or −264.5 J g −1 .
The more stable, normally present ( E ) -azobenzene has no dipole moment (µ = 0 D ), in contrast to the metastable ( Z ) -azobenzene (µ = 3 D).
Presentation and extraction
Azobenzene ( 5 ) can be produced in the following ways (see also the picture below):
- by reduction of nitrobenzene ( 1 ) with sodium amalgam or lithium aluminum hydride (route A )
- by oxidation of hydrazobenzene ( 2 ) with sodium hypobromite solution (route B )
- by condensation of nitrosobenzene ( 3 ) with aniline ( 4 ) in acetic acid solution (especially for the production of asymmetric derivatives ) (route C )
use
( E ) azobenzene is used as a test substance for the Kofler hot stage microscope or as a calibrator for the Kofler hot bench use.
literature
- H. Zollinger: chemistry of azo dyes . ( Chemical series , vol. 13). Birkhäuser Verlag, Basel, 1958.
Individual evidence
- ^ A b c d e Hans Beyer , Wolfgang Walter : Textbook of Organic Chemistry , 20th Edition, Hirzel, Stuttgart 1984. P. 529.
- ↑ a b c d Entry on azobenzene in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on Azobenzene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ E. Mitscherlich: Annalen der Physik und Chemie XXXII (1834), p. 224.
- ↑ E. Mitscherlich: Annalen der Chemie and Pharmacie XII, p. 311.
- ↑ Otto Linné Erdmann: Journal for practical chemistry . tape 82 . Publisher by Johann Ambrosius Barth, 1861, p. 444 ( read online in Google Book Search).
- ↑ P. Hofmann: Annals of Chemistry and Pharmacy CXV. P. 362.
- ↑ Peter Griefs: On the knowledge of the so-called azobenzene and related compounds . In: Justus Liebig, Freiherr von (Ed.): Annals of Chemistry and Pharmacy . Volumes 131-132. CF Winter'sche, 1864 ( read online in the Google book search).
- ↑ P. Hofmann: Annalen der Chemie und Pharmacie (1860), p. 324.
- ↑ Dr. Carl Glaser: About a new way of forming azobenzene . In: Journal of Chemistry . 1866 ( read online in Google book search).
- ↑ A. Hantzsch, G. Reddelien: Die Diazoverbindungen . Springer, Berlin, 1921.
- ↑ GS Hartley: Nature 140 (1937). P. 281.
- ↑ Robertson, JM: Crystal structure and configuration of the isomeric azobenzenes in J. Chem. Soc. 1939, pp. 232-236, doi : 10.1039 / JR9390000232 .
- ↑ Cook, AH: The preparation of some cis-azo compounds in J. Chem. Soc. 1938, pp. 876-881, doi : 10.1039 / JR9380000876 .
- ↑ a b Wolf, E .; Cammenga, HK: Thermodynamic and Kinetic Investigation of the Thermal Isomerization of Cis-azobenzene in Z. Phys. Chem. 107 (1977) pp. 21-38, doi : 10.1524 / zpch.1977.107.1.021 .
- ↑ a b Eckardt, N .; Flammersheim, HJ; Cammenga, HK: The cis-trans isomerization of azobenzene in the molten state in J. Therm. Anal. Calorim. 52 (1998), pp. 177-185, doi : 10.1023 / A: 1010178610642 .
- ↑ RJW Le Fevre, GS Hartley: The dipole moments of cis and trans azobenzenes and of some related compounds in J. Chem. Soc. 1939, pp. 531-535, doi : 10.1039 / JR9390000531 .
- ^ M. Kuhnert-Brandstätter: Thermomicroscopy in the Analysis of Pharmaceuticals, Pergamon Press, Oxford (1971).