Aniline black
Aniline black is one of the oldest synthetic colorants .
On the one hand, aniline black describes a dye that is produced by oxidation of aniline salts with potassium chlorate , bichromate and the like, almost always directly on the fiber ( cotton , more rarely on silk or half silk ). Aniline black is one of the most genuine and beautiful black dyes and is therefore of great importance , especially in cotton dyeing .
On the other hand, it was successfully used as a pigment in the paint sector, but was then replaced by the pigment soot that is dominant today and is now of little importance. Aniline black is the oldest synthetically produced organic pigment.
discovery
In 1834 Friedlieb Ferdinand Runge first observed the formation of a green dye when he allowed hydrochloric aniline to act on fabric treated with bichromate. The discovery by William Henry Perkin in 1856, which can also be found in the literature, is therefore incorrect. He discovered the "development" to pigment through the oxidation of aniline with potassium dichromate . It was not until 1863 that John Lightfoot Junior developed this process into a practical aniline black dyeing method by performing the oxidation in the presence of copper salts directly on the fiber. He later patented this discovery.
presentation
For the oxidation to proceed correctly according to Lightfoot, certain catalysts , oxygen carriers, are necessary, among which vanadium, copper and iron salts are the most preferred. Depending on how the oxidation is carried out, different oxidation states of the aniline black are formed.
When working in the cold, the so-called emeraldine can be isolated, which is green as a salt and blue as a base . According to Richard Willstätter , it is supposed to arise from the coming together of 8 aniline molecules and have two quinoid groups. With further oxidation it would change into the trichinoid stage, the nigraniline , and finally into the quadruple quinoid pernigraniline .
Emeraldine and nigraniline, and to a lesser extent pernigraniline, are very acid-sensitive, greenish. This behavior is in good agreement with the assumed quinone imine structure .
However, through oxidation in the heat in the presence of aniline , the technology produces a greenish aniline black that is almost entirely resistant to acids and reducing agents . A quinone imine formula does not do justice to this resilience.
According to Green, it is very likely that the greenish aniline black is formed from pernigraniline by condensation of the latter with three molecules of aniline, forming a phenylphenazonium salt with three phenazine rings .
structure
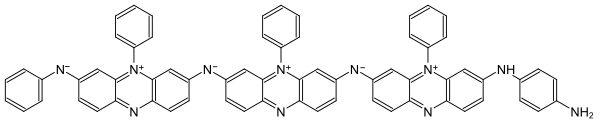
Since the synthesis of aniline black produces a reaction mixture of different dyes, it is not possible to clearly assign a structural formula. An example of a dye is shown below:
properties
The hue of aniline black is described as deep, neutral black. Aniline black has high hiding power and good dispersibility , but has a much lower color strength than carbon black . The pigment is slightly conductive. The light and weather resistance is in full shade well, but takes in white reductions sharply. CI Pigment Black 1 can influence the surface of paints and creates an appearance that is described as matt and velvety.
Sources of danger
| safety instructions | |||||||
|---|---|---|---|---|---|---|---|
| Surname |
|
||||||
| CAS number |
13007-86-8 |
||||||
|
|||||||
Depending on the quality, aniline black can contain different levels of aniline. This can have a carcinogenic effect and - in significantly larger quantities than occurs here - would be a blood poison.
use
Aniline black is used particularly in cotton dyeing . In other areas, aniline black is only used nowadays when pigment soot leads to problems or the matting of the surface is to be created in a targeted manner. It is used in the paint and printing inks sector when processing problems are caused by carbon black pigments. In the plastics sector, this is the case when problems arise during welding due to pigment soot.
Individual evidence
- ↑ H. Kittel, J. Spille: Textbook of paints and coatings. Volume 5: Pigments, Fillers and Colorimetry. 2nd Edition. Hirzel, Stuttgart 2003, ISBN 3-7776-1015-1 .
- ↑ a b c d W. Herbst, K. Hunger: Industrial organic pigments. 2nd Edition. VCH Verlagsgesellschaft, Weinheim 1987, ISBN 3-527-26319-5 .
- ^ C. Reinhardt, AS Travis: Heinrich Caro and the Creation of Modern Chemical Industry. Springer, 2000, ISBN 0-7923-6602-6 .
- ↑ Prof. Blume's media offer .
- ^ The Society of Dyers and Chemists: Color Index. Third edition; Second revision. Charlesworth & Co., Huddersfield 1982.
- ↑ a b H. Römpp: Römpp Lexikon; Lacquers and printing inks. Thieme, Stuttgart 1998, ISBN 3-13-776001-1 .
- ↑ There is not yet a harmonized classification for this substance . A labeling of Benzenamine, oxidized in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 9, 2016, is reproduced from a self-classification by the distributor .
literature
- Paul Karrer: Textbook of Organic Chemistry. 10th edition. Georg Thieme Verlag, Stuttgart 1948, p. 618.
- Carl Cramer: About aniline black. PhD thesis . 1911. (PDF file; 2.04 MB).
- Aniline black. In: Otto Lueger : Lexicon of the entire technology . at www.zeno.org.