Methyl red
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Methyl red | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 15 H 15 N 3 O 2 | ||||||||||||||||||
| Brief description |
red-purple solid with a faint odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 269.31 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
178-182 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Methyl red , also 4'-dimethylamino-azobenzene-2-carboxylic acid , is a water-insoluble dye from the group of azo dyes . The water-soluble sodium salt is an acid-base indicator .
properties
Methyl red is a red-purple solid with a faint odor.
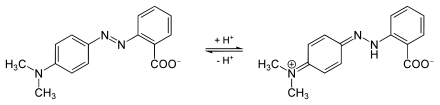
The color of aqueous methyl red solutions changes to red at a pH value of less than 4.4 and to yellow from 6.2. The solution is orange between pH 4.4 and 6.2.
synthesis
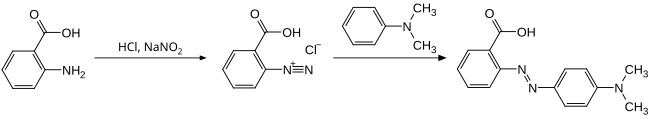
The synthesis proceeds via an azo coupling . To do this, the amino group of the anthranilic acid is first converted into a diazonium group with the help of sodium nitrite and hydrochloric acid and this is reacted with N , N -dimethylaniline . This reaction must take place in the cold because of the instability of the diazo compound.
use
In analytical chemistry , methyl red is an important indicator for the titration of strong acids with weak bases .
In microbiology , methyl red is used to stain bacteria for microscopic examinations that produce organic acids during the fermentation of glucose .
Individual evidence
- ↑ a b c d e f data sheet methyl red (CI 13020) (PDF) from Merck , accessed on May 9, 2017.
- ↑ External identifiers or database links for methyl red sodium salt : CAS number: 845-10-3, EC number: 212-682-9, ECHA InfoCard: 100.011.530 , GESTIS substance database : 107499 , PubChem : 4465632 , ChemSpider : 92048 , Wikidata : Q27255991 .
- ↑ Martha Windholz: The Merck index: an encyclopedia of chemicals and drugs . Ed .: Merck. 9th edition. Rahway NJ 1976, ISBN 0-911910-26-3 , pp. 798 .
- ^ Organikum authors' collective , 22nd edition, Wiley-VCH, Weinheim 2004, p. 645.