Congo red
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Congo red | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 32 H 22 N 6 Na 2 O 6 S 2 | ||||||||||||||||||
| Brief description |
red-brown odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 696.66 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
> 360 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Authorization procedure under REACH |
particularly worrying : carcinogenic ( CMR ) |
||||||||||||||||||
| MAK |
Germany: 4 mg · m -3 (measured as respirable dust ) and 0.3 mg · m -3 (measured as respirable dust ) |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
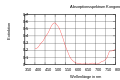
Congo belongs, as well as methyl orange and methyl red , to the group of azo dyes and will among other things as pH - indicator used. The color of Congo red changes from blue-violet to red-orange at pH 3.0 to 5.2. It is therefore suitable as an indicator for acid-base titration .
The structural formula shows Congo red as a disodium salt , in which both sulfonic acid groups (–SO 3 H) are deprotonated . The values in the table also relate to the salt.
history
Congo red was the first direct dye and was developed by Paul Böttiger at Bayer in 1883 . He later sold the patent to Agfa , who later entered a cartel with Bayer. A patent litigation for Congo red started in Berlin in 1889 introduced the patent feature of the technical effect (in this case the property of direct coloring).
The name Congo red was introduced in 1885 by Agfa (then in Berlin) for marketing reasons. The historical background is probably that the Congo Conference had just ended in Berlin and therefore the term Congo was on everyone's lips.
presentation
Congo red is obtained by tetrazotization of benzidine and coupling to 4-aminonaphthalene-1-sulfonic acid .
properties
The color of Congo red changes from blue-violet to red at a pH value of 3.0 to 5.2. The photometer shows an absorption maximum at approx. 500 nm (pH> 6) or 647 nm and 590 nm (pH <3).
use
As Congo paper is a designated indicator paper similar to litmus paper . It shows a color change from red to blue with a decreasing pH value from 5.0 to 3.0. It is used specifically for the detection of lactic acid , e.g. B. bacterial cultures (E. coli), to neutralization analysis as well as for the detection of acids , such as free hydrochloric acid .
In biology , Congo red is used e.g. B. used in feeding experiments. Protozoa are fed on yeasts stained with Congo red, and it is possible to observe the intake of food and the enzymatic digestion .
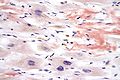
In pathology , Congo red is used to represent amyloid deposits that indicate amyloidosis .
In mycology , Congo red is a staining reagent for staining fungal hyphae. It is used as an aqueous solution in conjunction with ammonia or sodium dodecyl sulfate (SDS).
Staining of amyloid in the meningeal blood vessel wall in cerebral amyloid angiopathy
safety instructions
Since the carcinogenic benzidine can be released from Congo red under reductive conditions , this dye, like benzidine itself, is one of the forbidden substances according to the Consumer Goods Ordinance (BedGgstV) and must not be used for textile and leather products that have been in contact with human skin for a long time or the oral cavity (e.g. clothing, bed linen, towels, shoes, gloves, yarns and fabrics intended for end users).
Web links
Individual evidence
- ↑ a b c d e Entry on disodium 3,3 ′ - ((1,1′-biphenyl) -4,4′-diylbis (azo)) bis (4-aminonaphthalene-1-sulfonate) in the GESTIS substance database of the IFA , accessed January 8, 2018(JavaScript required) .
- ↑ External identifiers of or database links to Congo red : CAS number: 14684-01-6, PubChem : 11314 , ChemSpider : 21865348 , Wikidata : Q27104437 .
- ^ Dangerous substances database of the federal states , June 19, 2007.
- ↑ Entry on Disodium 3,3 ′ - [[1,1′-biphenyl] -4,4′-diylbis (azo)] bis (4-aminonaphthalene-1-sulphonate) in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA) , accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on October 18, 2015.
- ↑ Congo red data sheet (CI 22120) (PDF) from Carl Roth , accessed on November 9, 2015.
- ↑ Congo red data sheet (CI 22120) (PDF) from Merck , accessed on February 4, 2018.
- ↑ David P. Steensma: "Congo Red", Out of Africa? In: Archives of pathology & laboratory medicine. Volume 125, Number 2, February 2001, pp. 250-252, doi : 10.1043 / 0003-9985 (2001) 125 <0250: CR> 2.0.CO; 2 , PMID 11175644 . PDF
- ↑ Kehrer, Erwin: The physiological and pathological relationships of the female sexual organs to the intestinal tract and especially to the stomach (1905) (for the detection of hydrochloric acid with Congo paper). ( Digitized version ).
- ↑ Wissenschaft-online.de: Hopeful Contrast , accessed on June 25, 2007.
- ↑ Heinz Clemencon: Methods for Working with macrofungi . Ed .: IHW-Verlag, 2009. ISBN 978-3-930167-73-9 .
- ↑ Consumer Goods Ordinance, Appendix 1 (to Section 3). Substances that may not be used in the manufacture or treatment of certain commodities. Federal Ministry of Justice and Consumer Protection, accessed on October 29, 2019 .
- ^ Entry on benzidine-based azo dyes in the GESTIS substance database of the IFA , accessed on November 1, 2008(JavaScript required) .