Auxochrome

|
|
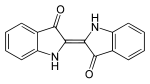 |

|
| Comparison of Indigo (left) and purple (right) in colorations: the two additional bromine - substituent in purple act hypsochromically . |
|
Auxochromes (from the Greek αυξειν auxein “grow” and chroma “color”) are functional groups in dye - molecules that shift the absorption maximum of an already existing coloring group ( chromophore ) into the longer-wave range of the spectrum . This results in a visually perceptible color change, which is referred to as the bathochromic effect . The absorption maximum of the chromophore alone is often in the UV range and is not visible to the human eye.
According to Witt's dye theory , a dye typically consists of a coloring group ( chromophore ) and one or more auxochromic , ie color-enhancing groups.
The effect of the auxochromes is caused by their effect as electron pair donors . These supply the conjugated system of the chromophore with electron density and thus support its delocalization, which means that lower-energy, i.e. longer-wave, light is required for excitation. Examples of auxochromes are functional groups with free electron pairs such as -OH , -NH 2 , -OR or -NR 2 , which cause a corresponding color change via a + M effect with the double bond system of the chromophore. Alkyl groups, which only act as electron pair donors via a + I effect, also belong to the auxochromes, although their effect is weaker.
The bathochromic effect can be enhanced by anti-luxochromes . These are functional substituents with an electron-withdrawing −M effect - or −I effect . Together with auxochromes they are coupled to the chromophore and intensify the delocalization of the electrons. Since the donor effect of the auxochromes is usually stronger, antiauxochromes do not counteract but support the bathochromic effect. Examples of antiauxochromic groups are the carbonyl or nitro group .
Hypsochromes are functional substituents that only have an −I effect. These substituents are the only functional groups that lead to a blue shift of the chromophore. The absorption maximum of an already existing chromophore is shifted into the shorter-wave range of the light spectrum. Hypsochromic groups are rarely used in a targeted manner and play a subordinate role, since the absorption spectrum, as mentioned above, is mostly in the UV range and has to be shifted into the visible range.
See also
literature
- Heinrich Zollinger, A. Iqbal: Color chemistry: syntheses, properties, and applications of organic dyes and pigments. 3. Edition. Helvetica Chimica Acta , 2003, ISBN 3-906390-23-3 , p. 19 ( referenced section in the Google book search).