Amidines
Amidines are a group of organic chemical compounds that can be formally described as derivatives of carboxamides in which the carbonyl group has been replaced by a carbimino group . The chemistry of amidines was fundamentally researched by Adolf Pinner (1842–1909).
structure
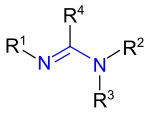
The non-cyclic amidines have the general structural formula R 4 -C (= NR 1 ) -NR 2 R 3 , where the radicals R are hydrogen atoms or organyl radicals .
The structurally simplest amidine with R 1 to R 4 = H is called formamidine and is derived from formic acid (Latin acidum formicum). When R 4 = CH 3 ( methyl group ) and R 1 to R 3 = H, it is the acetamidine derived from acetic acid .
As strong organic bases, the bicyclic amidines DBN and DBU are important in which both N atoms are components of the ring systems.
synthesis
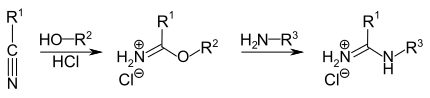
Amidines can be produced in a multi-step process using the Pinner reaction . This reaction starts from the corresponding nitriles R 1 -CN, which are activated with HCl and then converted with alcohols to form imidic acid esters , the Pinner salts. The amidines are formed from these salts by adding amines.
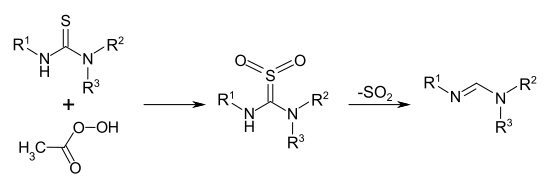
The production of formamidines as the derivatives of formic acid is not possible in this way, because in order to produce the pinner salts in question, the volatile and toxic hydrocyanic acid itself would have to be used instead of a nitrile . Therefore there are z. B. for the production of tri-substituted formamidines special methods that start from tri-substituted thioureas R – NH – C (= S) –NR 2 . The thioureas are converted into the relevant thiourea-SS-dioxides R-NH-C (= SO 2 ) -NR 2 by oxidation with peracetic acid . These unstable compounds split off sulfur dioxide SO 2 and thus the correspondingly substituted formamidines R – N = CH – NR 2 are formed .
Properties and use
Together with 1,3-diketones, amidines are the building blocks for the synthesis of pyrimidines .
With a pK value of 13.6, amidines are almost as strong bases as sodium hydroxide and form stable salts with acids. The sp2-hybridized imino group = NH is protonated with the formation of a mesomeric-stabilized cation , which can only form after protonation on the imino nitrogen .
The two bicyclic amidines DBU and DBN are also very strong bases and have another advantage for use in synthetic organic chemistry over other strong bases such as alkali hydroxides, alkali alcoholates or alkali hydrides : The large, voluminous bicyclic organic bases are good in organic solvents soluble and do not show nucleophilic properties, they are non-nucleophilic bases.
Although their molecules are very voluminous, they can attack and bind easily accessible protons. They are well suited to initiate elimination reactions (e.g. elimination of HCl from chloroalkanes with formation of alkenes ). However, the voluminous bases are not able to attack shielded carbon atoms nucleophilically, so that undesired substitution reactions do not occur.
Individual evidence
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 63.
- ↑ Wolfgang Walter , Klaus-Peter Rueß: About the oxidation products of thiocarboxamides, XXIV. Representation of the S. S-Di and S. S-S trioxides of trisubstituted thioureas and a new method for the preparative production of trisubstituted formamidines . In: Chemical Reports . tape 102 , no. 8 , August 1969, p. 2640-2650 , doi : 10.1002 / cber.19691020820 .
- ^ A b Clayden, Greeves Warren, Wothers: Organic Chemistry . Oxford University Press Inc, New York 2001, ISBN 978-0-19-850346-0 , pp. 202, 482 .