Pinner reaction
The Pinner reaction is a name reaction in organic chemistry . The main application of the Pinner reaction is in the production of carboxylic acids , esters , thioesters and amides . It is named after the German chemist Adolf Pinner (1842–1909).
Overview
The starting material for all end products of the Pinner reaction is a so-called Pinner salt. This is made from a nitrile and an alcohol under the action of hydrochloric acid and produces the Pinner salt, the hydrochloride of an imidate .
The Pinner salt can be used as a starting material for the synthesis of many organic compounds - carboxylic acid esters , orthoesters or protonated amidines .
mechanism
Making a pinner salt
In this reaction, a Pinner salt ( 4 ) is first generally produced. This salt can then be processed further by reacting it with other reagents.
First, a nitrile 1 is activated with dry hydrochloric acid (HCl gas) so that the protonated form 2 is present. An alcohol can now attack the carbon atom of the nitrile group nucleophilically. The electrons of the CN triple bond of the nitrile rearrange and the imino group 3 is formed. This is followed by an intramolecular proton transfer and the cation of the so-called Pinner salt 4 (with chloride as the anion) is formed.
Production of esters and amidines from Pinner salts
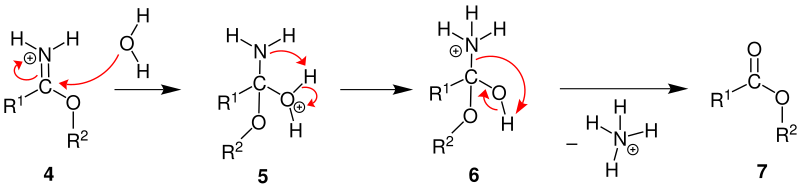
If the Pinner salt is mixed with water, a carboxylic acid ester can be obtained from it . If no alcohol is stored on the nitrile, as described above, but a thiol or an amine , thioesters or amidines can also be prepared.
If the Pinner salt ( 4 ) is mixed with water, an oxonium ion 5 is formed . Then an intramolecular proton transfer takes place between the oxonium group and the amino group. This creates an ammonium with a hydroxyl group 6 . By rearranging one pair of electrons, the oxygen in the water that has just been added deprotonates and forms a carbonyl group. In addition, ammonia is split off from the molecule. This absorbs the hydrogen ion that was released during the deprotonation. In total, one ammonium ion leaves the molecule. This forms a carboxylic acid ester 7 .
Preparation of a 1-amino-1-iminium salt ( amidinium salt )
If you work up a Pinner salt with an amine , an iminium salt with an amino group is formed .

If a primary or secondary amine is added to a Pinner salt ( 4 ), the nitrogen particle of the amine attaches to the carbon of the Pinner salt. This is how molecule 8 is formed. The amine that has just been deposited deprotonates , resulting in intermediate 9 . By rearranging a pair of electrons , a double bond is formed again between the nitrogen and the carbon atom. Furthermore, the electron pair is rearranged between the carbon and oxygen atoms. As a result, an alcoholate anion leaves the molecule. This is very basic, which is why it reacts with the previously split off proton to form an alcohol. These steps form the amidinium salt 10 , with a chloride ion being the counterion.
Individual evidence
- ↑ a b c B. P. Mundy, MG Ellerd, FG Favaloro: Name Reactions and Reagents in organic Synthesis , 2nd edition, Wiley-Interscience, Hoboken, NJ 2005 , ISBN 978-0-471-22854-7 , p. 516.