1,4-thiazine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
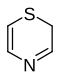
|
||||||||||
| 2 H -1,4-thiazine | ||||||||||
| General | ||||||||||
| Surname | 1,4-thiazine | |||||||||
| Molecular formula | C 4 H 5 NS | |||||||||
| Brief description |
colorless liquid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 99.15 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| boiling point |
76.5-77 ° C |
|||||||||
| pK s value |
5.6 |
|||||||||
| solubility |
miscible with water |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
1,4-thiazine is the simplest chemical compound from the group of thiazines .
Extraction and presentation
1,4-Thiazine can be obtained by reducing the imide of mercaptoacetic acid with aluminum powder at elevated temperatures.
properties
1,4-thiazine is a colorless liquid that is miscible with water and has an ammoniacal odor with a slight additional smell of sauerkraut. It forms stable salts with picric acid , hydrogen chloride and hexachloroplatinic acid .
From the compound theoretically exist three tautomeric forms and although 4 H - 2 H -1,4-thiazine and 1λ 4 , 4-thiazine. Most of the derivatives of 1,4-thiazine are in the 2 H form, which also applies to the parent compound. The most important 4 H derivatives are the phenothiazines , which are used in pharmacology and dye chemistry. From 1λ 4 , from 4 to 1.4-thiazine few derivatives are known.
Individual evidence
- ↑ a b c d e f Charles Barkenbus, Phillip S. Landis: The Preparation of 1,4-Thiazine. In: Journal of the American Chemical Society . 70, 1948, pp. 684-685, doi : 10.1021 / ja01182a075 .
- ↑ a b c D. Angerhöfer, Heinz Dehne, Günther Klar, Harun Parlar, Wolf-Diethard Pfeiffer: Houben-Weyl Methods of Organic Chemistry Vol. E 9a, 4th Edition Supplement Hetarenes IV (Six-Membered Rings and Larger Hetero-Rings with Maximum unsaturation) . Georg Thieme Verlag, 2014, ISBN 3-13-181484-5 , p. 428 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Entry on thiazines. In: Römpp Online . Georg Thieme Verlag, accessed on May 22, 2019.
- ^ AR Katritzky, AJ Boulton: Advances in Heterocyclic Chemistry . Academic Press, 1981, ISBN 978-0-08-057611-4 , pp. 296 ( limited preview in Google Book search).

