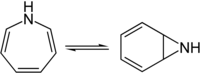
1 H -azepine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| General | ||||||||||
| Surname | 1 H -azepine | |||||||||
| other names |
Azatropilids |
|||||||||
| Molecular formula | C 6 H 7 N | |||||||||
| Brief description |
red liquid (at −78 ° C) |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 93.13 g mol −1 | |||||||||
| pK s value |
11.07 |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Azepine is an unsaturated heterocyclic chemical compound . It is the simplest seven-membered unsaturated nitrogen- containing heterocycle.
Manufacturing
The synthesis of 1 H -azepin can be carried out by decomposition of the azepine-1-carboxylic acid. This is accessible from a ring expansion analogous to the Buchner reaction from benzene and an azido formic acid ester.
properties
1 H -azepine is in equilibrium with the isomeric aziridine derivative of benzene.
The azepine cation is aromatic because it has a fully conjugated system of six π electrons.
1 H -azepine is unstable at room temperature and rearranges into the more stable 3 H -azepine.
Reactions
Bases catalyze the tautomerization of 1 H -azepine to 3 H -azepine.
Individual evidence
- ↑ a b c Entry on Azepine. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b E. Vogel, H.-J. Altenbach, J.-M. Drossard, H. Schmickler, H. Stegelmeier: 1 H -Azepine: NMR spectroscopic and chemical characterization , in: Angew. Chem. 1980 , 92 , 1053-1054; doi : 10.1002 / anie.19800921221 .