Buchner reaction
The Buchner reaction is a name reaction in organic chemistry in which ethyl diazoacetate reacts thermally or photochemically with benzene or its homologous compounds to form the corresponding isomeric esters of cycloheptatriene . This implementation was first published in 1885 by the German chemists Eduard Buchner (1860–1917) and Theodor Curtius (1857–1928) and expanded decades later by the American chemist William von Eggers Doering and his colleagues.
Overview reaction
In the Buchner reaction, a six-membered aromatic (e.g. benzene) reacts to form a seven-membered ring (e.g. cycloheptatriene).
Reaction mechanism
In the proposed reaction mechanism, according to Buchner, the diazoacetic ester 1 reacts with benzene to form norcaradiene 2 with elimination of nitrogen and formation of a carbene . William von Eggers Doering and his co-workers were able to use modern NMR technology to demonstrate that norcaradiene 2 is only an intermediate product in the synthesis and that it reacts further in a 6 -ring opening to give cycloheptatriene 3 . 2 and 3 exist in a dynamic equilibrium, with the formation of cycloheptatriene 3 being strongly favored.
This cycloheptatriene 3 is subject to a [1,5] hydride shift, so that the isomers 4 - 6 of cycloheptatriene be formed. Modern NMR technology shows that the product is a mixture of cycloheptatrienes 4 to 6 , which are also known as “Buchner esters”.
application
The Buchner reaction can be found in an intermediate step in the synthesis of a drug called Egualen Sodium , which works against ulcers . The product is a substituted azulene . But the Buchner reaction is not only used for the synthesis of this medicinal substance . The diterpenoid tropone called harringtonolide and fullerene derivatives can also be synthesized for medical use using the Buchner reaction.
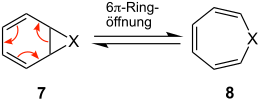
Heterocycle Synthesis
The ring expansion is not limited to the synthesis of carbocycles (X = CR 2 ), but can be transferred to heterocycles (X = NR, O):
The position of the equilibrium between 7 and 8 depends on the nature of X.
Individual evidence
- ↑ a b László Kürti , Barbara Czakó: Strategic applications of named reactions in organic synthesis: background and detailed mechanisms . Elsevier Academic Press, Amsterdam 2005, ISBN 0-12-429785-4 , pp. 68-69 .
- ↑ a b E. Buchner, Th. Curtius: About the action of diazoacetic ether on aromatic hydrocarbons . In: Reports of the German Chemical Society . tape 18 , no. 2 , 1885, p. 2377-2379 , doi : 10.1002 / cber.188501802119 .
- ↑ a b c W. by E. Doering, G. Laber, R. Vonderwahl, NF Chamberlain, RB Williams: THE STRUCTURE OF THE BUCHNER ACIDS . In: Journal of the American Chemical Society . tape 78 , no. October 20 , 1956, p. 5448 , doi : 10.1021 / ja01601a080 .
- ^ A b Sarah E. Reisman, Roger R. Nani, Sergiy Levin: Buchner and Beyond: Arene Cyclopropanation as Applied to Natural Product Total Synthesis . In: Synlett . tape 2011 , no. October 17 , 2011, p. 2437-2442 , doi : 10.1055 / s-0031-1289520 .
- ↑ John L. Kane, Kevin M. Shea, Aimee L. Crombie, Rick L. Danheiser: A Ring Expansion - Annulation Strategy for the Synthesis of Substituted Azulenes. Preparation and Suzuki Coupling Reactions of 1-Azulenyl Triflates . In: Organic Letters . tape 3 , no. 7 , 2001, p. 1081-1084 , doi : 10.1021 / ol0156897 .
- ↑ Roberto Pellicciari, Danilo Annibali, Gabriele Costantino, Maura Marinozzi, Benedetto Natalini: Dirhodium (II) Tetraacetate-Mediated Decomposition of Ethyldiazoacetate and Ethyldiazomalonate in the Presence of Fullerene. A New Procedure for the Selective Synthesis of [6-6] Closed Methanofullerenes . In: Synlett . tape 1997 , no. 10 , October 1997, p. 1196-1198 , doi : 10.1055 / s-1997-980 .
- ↑ Barbara Frey, Adam P. Wells, Daniel H. Rogers, Lewis N. Mander: Synthesis of the Unusual Diterpenoid Tropones Hainanolidol and Harringtonolide . In: Journal of the American Chemical Society . tape 120 , no. 8 , 1998, pp. 1914–1915 , doi : 10.1021 / ja9738081 .
- ^ Ulrich Lüning: Organic reactions , 2nd edition, Elsevier GmbH, Munich 2007, ISBN 978-3-8274-1834-0 , p. 169.