Imidazolines
Imidazolines are a chemical group from the field of heterocyclic compounds and belong to the group of azolines . Its members are five-membered cyclic organic unsaturated compounds that have exactly two non-adjacent nitrogen atoms in the ring structure. They are derived from the imidazoles by hydrogenation of a double bond and are isomeric to the pyrazolines . There are three basic structures of the imidazolines, which are isomeric to one another and differ in the position of the double bond.
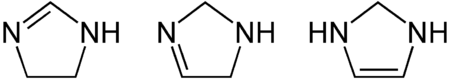
Imidazolines can form stable carbenes . For this purpose, imidazoles substituted on both nitrogen atoms can be reduced with sodium hydride and potassium tert -butanolate . The resulting carbenes are stable and are called Arduengo carbenes . These carbenes can be used, for example, as ligands on Grubbs catalysts .
Web links
swell
- ^ H. Beyer, W. Walter: Textbook of Organic Chemistry , 23rd Edition, S. Hirzel Verlag, Stuttgart 1998, pp. 795-896, ISBN 3-7776-0808-4 .
- ↑ M. Scholl, S. Ding, CW Lee, RH Grubbs: Synthesis and Activity of a New Generation of Ruthenium-Based Olefin Metathesis Catalysts Coordinated with 1,3-Dimesityl-4,5-dihydroimidazol-2-ylidene Ligands , in: Organic Letters 1999 , 1 , 953-956; doi: 10.1021 / ol990909q .

