meta -chloroperbenzoic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
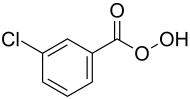
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | meta -chloroperbenzoic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 5 ClO 3 | |||||||||||||||
| Brief description |
white, moist powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 172.57 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.56 g cm −3 |
|||||||||||||||
| Melting point |
69-71 ° C |
|||||||||||||||
| boiling point |
Decomposes at 88 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
meta -Chlorperbenzoesäure often abbreviated as, mCPBA ( English m - c hloro p eroxy b enzoic a cid ) or MCPBA referred to is a peroxycarboxylic acid .
Extraction and presentation
meta -chloroperbenzoic acid can be prepared by reacting 3-chlorobenzoyl chloride ( m -chlorobenzoyl chloride) with hydrogen peroxide in the presence of magnesium sulfate and aqueous sodium hydroxide solution in dioxane , followed by acidification with dilute sulfuric acid.
properties
Like all peroxycarboxylic acids, it has a strong oxidizing effect.
mCPBA with a content of about 75% is commercially available in a mixture of water and 3-chlorobenzoic acid. In this mixture, mCPBA has proven to be harmless, while the pure peracid is explosive and is sensitive to impact and higher pressures or shear forces. The peracid can be purified from this mixture by washing with a slightly basic buffer and then drying. Peracids are far less acidic than the actual carboxylic acid , so that the latter are removed during the washing process. The peracid purified in this way must be stored in a plastic container at a low temperature.
use
mCPBA is used in organic chemistry for oxidation reactions. Safe and easy handling often make mCPBA the reagent of choice. The main area of application is in reactions such as the Baeyer-Villiger oxidation , the epoxidation of alkenes ( Prileschajew reaction ), the oxidation of sulfides to sulfoxides or sulfones and of amines to amine- N -oxides .
MCPBA is very soluble in dichloromethane . Epoxidations of alkenes are preferably carried out at room temperature in this solvent; the m -chlorobenzoic acid released is insoluble in dichloromethane.
Individual evidence
- ↑ a b c d e Entry on 3-chloroperbenzoic acid in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Data sheet 3-Chloroperoxybenzoic acid from Acros, accessed on April 28, 2017.
- ↑ RN McDonald, RN Steppel, JE Dorsey: m-Chloroperbenzoic Acid In: Organic Syntheses . 50, 1970, p. 15, doi : 10.15227 / orgsyn.050.0015 ; Coll. Vol. 6, 1988, p. 276 ( PDF ).
- ↑ Purifiction of Laboratory Chemicals, 4th Ed., Oxford Butterworth-Heinemnn. 145, ISBN 0-7506-3761-7 .


