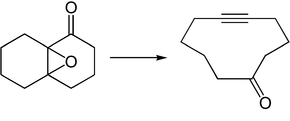
Eschenmoser fragmentation
The Eschenmoser fragmentation (often also called Eschenmoser-Ohloff or Eschenmoser-Tanabe fragmentation) is a name reaction in organic chemistry . It describes the synthesis of alkynes and ketones from α, β-unsaturated ketones or α, β-epoxy ketones derived therefrom . Either acid or base catalysis is used (here base catalysis with sodium carbonate ) and arylsulfonic acid hydrazide is required. The reaction is named after its discoverer Albert Eschenmoser and emerged from his collaboration with Firmenich researchers led by Günther Ohloff on the synthesis of muscon and other macrocylic musk bodies .
mechanism
The required α, β-epoxy ketones 1 are usually synthesized by Weitz-Scheffer epoxidation of α, β-unsaturated ketones. In the first steps condenses an arylsulfonic acid hydrazide (see first reaction arrow), for example, p -Toluolsulfonylhydrazid, with the epoxyketone 1 to form the sulfonyl hydrazone 4 . The subsequent fragmentation step can then be initiated with acid or base catalysis , with either the epoxide oxygen being protonated or the sulfonamide nitrogen being deprotonated. In both cases this results in compound 6 . In this mechanism, however, base catalysis is used with sodium carbonate . Typically, however, the process is acid-catalyzed with glacial acetic acid in dichloromethane at -18 ° C. With elimination of nitrogen and arylsulfinic acid, intermediate 6 fragments to give ketone 7 and alkyne 8 . The driving force behind the reaction is the formation of molecular nitrogen.
There is also a radical variant of this α, β-enone → alkynone fragmentation with 1,2-dibromo-5,5-dimethylhydantoin (DDH) in sec- butanol, which works without epoxidation and directly from the α, β-unsaturated hydrazone goes out. Allyl bromination with DDH takes place on the sulfonamide nitrogen, the capto-dative-stabilized radical site, and the bromide ion acts as a leaving group in the subsequent nucleophilic attack by an alcoholate ion. This Fehr- Ohloff- Büchi variant of the Eschenmoser-Ohloff fragmentation thus bypasses the epoxidation step, which often leads to poor yields of the classic Eschenmoser fragmentation in the case of sterically demanding substrates. By using bicyclic epoxy ketones, which carry the epoxide on both bridgeheads, cyclic 1,6-ynones are obtained, which is important for the synthesis of macrocycles .
Individual evidence
- ↑ J. Schreiber et al.: The synthesis of acetylene-carbonyl compounds by fragmentation of α, β-epoxy ketones with p-toluenesulfonylhydrazine. Preliminary communication. In: Helvetica Chimica Acta. 50, No. 7, 1967, pp. 2101-2108, doi : 10.1002 / hlca.19670500747 .
- ↑ Dorothee Felix, J. Schreiber, G. Ohloff, A. Eschenmoser: α, β-Epoxyketon → Alkinon-Fragmentierung I: Synthesis of exalton and rac-muscon from cyclododecanone via synthetic methods, 3rd part. In: Helvetica Chimica Acta. 54, No. 8, 1971, pp. 2896-2912, doi : 10.1002 / hlca.19710540855 .
- ^ Z. Wang: Comprehensive Organic Name Reactions and Reagents. Volume 1, Wiley, 2009, ISBN 978-0-471-70450-8 (3-Volume Set), p. 1005.
- ^ Charles Fehr, Günther Ohloff, George Büchi: A New α, β-Enone → Alkynone Fragmentation. Syntheses of exaltone® and (±) -muscone. In: Helvetica Chimica Acta. 62, No. 8, 1979, pp. 2655-2660, doi : 10.1002 / hlca.19790620815 .