Albert Eschenmoser
Albert Jakob Eschenmoser (born August 5, 1925 in Erstfeld / Switzerland ) is a Swiss chemist .
Life
Albert Eschenmoser, second-born son from Balgach in the St. Gallen Rhine Valley to parents, Alfons Otto Eschenmoser (1887–1977) and Johanna, born. Oesch (1894–1978), a citizen of Balgach, spent his youth in Erstfeld ( Canton Uri ), where his father worked as a butcher. He attended secondary school at the Kollegium St. Borromäus in Altdorf UR , the upper secondary school at the canton school in St. Gallen with a high school diploma in mathematics and natural sciences, studied natural sciences with a major in chemistry at the ETH Zurich and received his diploma in 1949 (dipl.sc. nat . ETH).
He received his doctorate in 1951 at Leopold Ruzicka's institute in the working group of Hans Schinz with the dissertation topic “On the acid-catalyzed cyclization of mono- and sesquiterpene compounds”. By order of Ruzicka, he was then able to continue the research started in the doctoral thesis with his own group of doctoral students.
In 1956 he became a private lecturer and in 1960 an associate professor. From 1965 until his retirement in 1992, Eschenmoser was a full professor of general organic chemistry at ETH Zurich.
After his retirement he continued his research on a small scale with postdocs: at the ETH Zurich until 2000, from 1993 to 1996 also as a guest in Christian Noe's laboratory at the Biozentrum of the University of Frankfurt / Main in collaboration with Gerhard Quinkert , and from 1996 to 2009 as professor at the Scripps Research Institute in La Jolla , where he led a research group together with his former ETH postdoctoral fellow Ramanarayanan Krishnamurthy.
Eschenmoser was visiting professor at the Massachusetts Institute of Technology , Cambridge MA (1961), at the University of Wisconsin – Madison (1965), at the Technion in Haifa (1969), at the University of Chicago (1970), Cambridge University , UK (1980) , Harvard University , Cambridge MA (1984), TH Darmstadt (1993), Johann Wolfgang Goethe University Frankfurt am Main (1994), Université catholique de Louvain / Katholieke Universiteit Leuven (1996) and the University of Vienna (2001).
Since 1954 Albert Eschenmoser has been with Elisabeth, geb. Baschnonga, married. He is the father of a daughter (Esther, 1961) and two sons (Jürg, 1958) and (Philipp, 1963). He has lived in Küsnacht ( Canton of Zurich ) since 1963 .
research
Eschenmoser was active in his research in various areas of organic and bio- organic chemistry . The main ones are: Terpene - biogenesis , mechanism and stereochemistry of organic-chemical reactions, development of new reactions and methods for organic synthesis, total synthesis of complex natural products , chemistry of hydroporphinoid ligand systems, etiology of the vitamin B 12 structure, etiology of the structure type of natural nucleic acids , and finally problems of prebiotic chemistry .
Terpene biogenesis
In his doctoral thesis , Eschenmoser suggested that the structural formulas of all cyclic sesquiterpenes known up to that point could be formally derived from a common aliphatic precursor by cyclization and rearrangement steps. He demonstrated the importance of the postulate by proposing new structural formulas for the sesquiterpenes zingiberen, β- caryophyllene , cloven, α-caryophyllene ( humulene ), cedren, elemol and lanceol. He subsequently extended this postulate to include the hypothesis of the oxidative initiation of a cationic cyclization of the triterpene hydrocarbon squalene . The constitutional formulas of all tetra- and pentacyclic triterpenes known at the time were thus derived from the formula of this aliphatic C 30 precursor. These formal-mechanistic relationships formed the occasion and basis for the formulation of the biogenetic isoprene rule by Leopold Ruzicka in 1953. With the help of a set of stereoelectronic and conformational rules about the steric course of cationic polyene cyclizations and rearrangements, Eschenmoser and Duilio Arigoni succeeded in deriving it in 1955 not only of the constitution , but also of the configuration of all the tetra- and pentacyclic triterpenes that were structurally known at the time. The biogenetic isoprene rule became an important instrument for elucidating the structure and biosynthesis of terpenoid natural products.
Mechanism and stereochemistry of organic chemical reactions
Eschenmoser's reaction mechanistic - stereochemical investigations include, in addition to the early work on the steric course of cationic polyene cyclizations in the terpene series, an experimental study on the relative rate of oxidation of secondary alicyclic alcohols to ketones with chromic acid . This study demonstrated that the difference in the rate of oxidation of epimeric secondary alcohols is not due to different steric hindrances to proton elimination , but to the reduction in steric tension during the transition from the starting material to the product. A work published in 1970 on nucleophilic substitution reactions on tetrahedral carbon indicated for the first time that endocyclic substitutions of the S N 2 type, in which the linear arrangement of nucleophile, substitution center and leaving group is not possible in the transition state of the reaction, are prohibited for stereoelectronic reasons. To Eschenmoser stereochemical studies include work on slow nitrogen inversion in N chloro aziridines and N alkoxy-1,2- oxazolidines .
Development of new reactions and synthetic methods
A multiply recurring in Oevre Eschenmoser's research topic is the reaction of the anionic type C, C-fragmentation as a method of regioselective formation of olefin - double bonds and C≡C- triple bonds . The conception and first targeted application of this type of reaction in an olefin synthesis go back to 1952. Later contributions to the topic are u. a. the α, β-epoxy ketone → alkynone fragmentation and the decarboxylative double fragmentation of bicyclic δ-tosyloxy carboxylates. Another focus was the chemistry of vinyl nitrosonium ions as highly electrophilic reactants. Work on the shift ( umpolung ) of nucleophilic reactivity from the α- to the β-position of conjugated enone systems by oximation of the carbonyl group dates from 1958 and 1965.
The developments that have been made in the course of the synthesis of vitamin B 12 include: the reductive C, C fragmentation of Diels-Alder adducts α, α, β, β-tetraesters, C, C linkage through iminoester / enamine - condensation and by means of the method of "sulfide contraction", the amide acetal variant of the Claisen rearrangement , which made accessible in crystallized form Mannich salt N , N -dimethyl N methylidene-ammonium iodide as methylenation - and methylation - Reagent , and finally the hydrolysis of a primary carboxamide group selective towards methoxycarbonyl groups by means of a vinylnitrosonium salt.
The epoxy ketone → alkynone fragmentation , the amide acetal variant of the Claisen rearrangement , the C, C coupling reaction by sulfide contraction, and the methylenation reagent all bear Eschenmoser's name (see also).
Synthesis of natural products
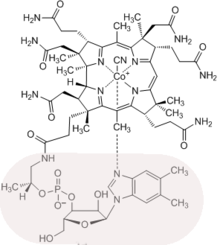
The main contribution Eschenmoser in the field of total synthesis of natural products is in addition to the synthesis of the alkaloid of the autumn crocus , colchicine , in collaboration with Robert B. Woodward ( Harvard University ) in Cambridge ( Massachusetts performed) synthesis of vitamin B 12 . This most complex of all vitamins contains a corrin ligand system as a central element , which differs from the biogenetically related tetrapyrrolic ligand systems of porphyrins (e.g. heme ) and chlorophylls through the structure of the chromophore , its peripheral boundary with C-methyl groups, and through the direct linkage the rings A and D are different. At the ETH Zurich , in model studies in 1964 and 1968, respectively, two different synthetic routes were developed for the synthetic structure type of the corrin ligand system of the vitamin, which had previously not been explored. At Harvard and ETH in 1972 two different syntheses of cobyric acid (and thus implicitly of vitamin B 12 ) were completed simultaneously. The strategies of the two cobyric acid syntheses correspond to the strategies of the two Corrin model syntheses and differ mainly in the construction of the structural part that contains the stereochemically complex direct link between rings A and D. In one variant, mainly worked on by the Harvard team, a strategically complex structure of this structural part is at the center of the synthesis. In the ETH variant, this structural part is created stereoselectively in a novel photochemical ring-closing reaction (A / D-Secocorrin? Corrin cycloisomerization). What both variants have in common is the structure of the Corrin-Chromophore system through multiple use of a new method of C, C linkage (sulfide contraction) developed in the course of the synthesis project. These two cobyric acid syntheses, comprising around 60 and 40 reaction steps respectively, were the result of a collaboration between two laboratories, which is unique for research in the field of organic chemistry, with around a hundred postdocs and doctoral students working in Cambridge and Zurich over the course of around 12 years .
After completion of the synthesis project, a systematic search for "dark variants" of the photochemical A / D secocorrin → cyclosisomerization began at the ETH with regard to the then still open question of the origin of the A / D structural part in the biosynthesis of vitamin B 12 . A whole series of such alternative (A → D) cyclizations were discovered, which revealed that there is a particularly easily constituting structural element of the vitamin B 12 molecule in the A / D structural part : the opposite of what was originally assumed ! This marked the beginning of experimental investigations into the etiology of the vitamin B 12 structure. These investigations extended, among other things, to structural types of new hexahydroporphinoid ligand systems whose chromophore systems correspond to those of corrin : corphin and pyrrocorphine. Hexahydroporphinoids of this type gained central importance in the research into the biosynthesis of vitamin B 12 from uroporphyrinogen III, which was just started in other laboratories .
A new type of dihydrocorphinoid ligand system was discovered during the elucidation of the structure of the nickel-containing coenzyme F430 from methanogenic bacteria , carried out at the ETH in collaboration with the microbiologist Rudolf Thauer in Marburg . A model compound of this ligand system was synthesized using corrin synthetic methods.
Etiology of the vitamin B 12 structure
Experiments on the etiology of a biomolecular structure start from the question: “Why is a biomolecular structure constituted in this way and not otherwise?” Questions of this kind can trigger experiments that lead to an approximation of an answer. The observations made in Eschenmoser's studies on the etiology of the B 12 structure relativize the high complexity of the structure according to formal criteria through the experimental evidence that the most important structural elements of vitamin B 12 (carbon / nitrogen skeleton, chromophore system , dimensions of the macrocyclic corrin - Rings, patterns of the peripheral C-methyl groups, attachment of the nucleotide chain) have a pronounced self-constitution potential. Chromophore system: According to model studies, the thermodynamic equilibrium between the hexahydroporphinoid ligand systems porphyrinogen and pyrrocorphine is on the porphyrinogen side of the free ligands; However, the latter isomerizes in the presence of metal ions via extensive tautomerization to give corresponding metal complexes of pyrrocorphine. Corrin complexes are thermodynamically more stable than corphinoid complexes of the same chromophore structure: this proves a thermally triggered ligand system contraction of a 20-hydroxy-20-methyl-19,20-dihydrocorphinate coordinated with either nickel (II) or dicyano-cobalt (III) to the corresponding 19-acetyl corrinate. Dimension of the corrin ring: The different dimensions of the coordination spaces of corphinoid (16-membered ring) and corrinoid (15-membered ring) ligand systems have different adhesive strengths of axial ligands in corresponding Ni (II) and dicyano-cobalt (III) complexes As a result: Axially coordinated ligands in corrinoid metal complexes are more labile than in corresponding corphinoid complexes. This difference is relevant for the function of coenzyme B 12 in terms of the question: “Why is vitamin B 12 a corrin and not a corphin?” Periphery of the macrocycle: conversion of zinc or magnesium pyrrocorphinates with methylating agents leads to C-methylation of the macro ring periphery with a regioselectivity that is reminiscent of the methylation pattern present in the B 12 structure. Attachment of the nucleotide: The attachment point on the propionic acid side chain in ring D, which is characteristic for the structure of vitamin B 12 , is the thermodynamically most stable of all four constitutionally analogous attachment options, or the one that forms selectively under suitable nucleotidation conditions.
Etiology of the structure type of natural nucleic acids
Finally, Eschenmoser transferred the question “Why this way and not differently?” On which the investigations into the etiology of the B 12 structure is based to the structure type of natural nucleic acids : “Why pentose - and not hexose - nucleic acids ? Why furanose - RNA and not pyranose - RNA ? ”. The synthesis and systematic examination of the mating behavior synthetic and potentially also natural variants of the structural type of natural nucleic acids revealed the fact that the Watson - Crick `specific base pairing any specific property of DNA and RNA: Alternative systems such. B. homo-DNA, pyranose-RNA ("p-RNA") or threose nucleic acid ("TNA") are also oligomer systems that carry information . Some of them even show stronger base pairing than RNA, e.g. B. “p-RNA”, which is made up of the same building blocks as RNA, but contains the ribose building block in the pyranose instead of the furanose form. Duplexes of “p-RNA” base sequences have a quasi-linear structure and not a helical one like RNA. As a result, “p-RNA” does not cross-pair with RNA. The "TNA", on the other hand, in which the sugar building block is present as furanose and which has a similar spatial structure to RNA, shows a pairing behavior similar to that of RNA and also the ability to pair informationally with both RNA and DNA. As an example of a non-enzymatic, chiroselective self -constitution of oligonucleotide duplexes, it was shown for the “p-RNA” that tetranucleotide '2', 3 '-cylophosphates of this type have a half-complementary sequence of the four nucleobases (e.g. ATCG ) are able to spontaneously oligomerize to duplexes of higher oligomers with a specific base sequence.
Prebiotic chemistry
In prebiotic chemistry , the possible abiotic formation of important biomolecules such as nucleotides , sugars and amino acids is a central topic. Eschenmoser's experimental contributions to this are the chemistry of α-amino-acrylonitrile and the 2- cyanoaziridine which forms photochemically from it , and above all the chemistry of glycolaldehyde phosphate, its formation from glycolaldehyde in highly diluted aqueous solution with amido triphosphate, and Evidence of the preferred formation of rac- ribose-2,4-diphosphate during its aldolization under basic conditions in the presence of formaldehyde . In his suggestion of a “glyoxylate scenario”, Eschenmoser pointed out a possibly central role of glyoxylic acid and its “dimers” dihydroxy-fumaric acid in biogenesis.
Awards (selection)
In total, Eschenmoser has received around 30 national and international academic prizes, including the following:
- Ruzicka Prize , ETH Zurich 1958
- Marcel Benoist Prize , Federal Department of the Interior , Bern 1973
- Robert A. Welch Award , RA Welch Foundation, Houston TX 1974
- August Wilhelm von Hofmann memorial coin , Society of German Chemists 1976
- Robert Robinson Award 1976
- Davy Medal , Royal Society , London 1978
- Tetrahedron Prize , Pergamon Press, London 1981
- Arthur C. Cope Award , American Chemical Society 1984
- Wolf Prize in Chemistry , Wolf Foundation, Israel 1986
- Cothenius Medal , German Academy of Sciences Leopoldina , Halle 1991
- Paracelsus Prize , Swiss Chemical Society 1999
- Grande médaille de l'Académie des sciences , Académie des Sciences , Paris 2001
- Roger Adams Award 2003
- Sir Derek H. Barton Gold Medal , Royal Society of Chemistry , London 2004
- Benjamin Franklin Medal , Franklin Institute , Philadelphia 2008
- Paul Karrer Medal , University of Zurich 2008
Albert Eschenmoser is Dr. hc from the Universities of Friborg , Chicago , Edinburgh , Bologna , Frankfurt / Main , Strasbourg , Harvard , Innsbruck and the Scripps Research Institute ( La Jolla , CA, USA).
Memberships (selection)
- American Academy of Arts and Sciences , Boston MA (Foreign Member, 1966)
- National Academy of Sciences , Washington, DC (Foreign Associate, 1973)
- German Academy of Sciences Leopoldina , Halle (member, 1976)
- Pontificia Academia Scientiarum , Stato della Città del Vaticano (member, 1986)
- Academy of Sciences in Göttingen (corresponding member, 1986)
- Royal Society , London (Foreign Member, 1986)
- Academia Europaea , London (Founding Member, 1988)
- Order Pour le mérite for Sciences and Arts , Berlin (foreign member , 1992)
- Austrian Decoration of Honor for Science and Art , Vienna (foreign member , 1993)
- Hrvatska akademija znanosti i umjetnosti , Zagreb , Croatia (corresponding member, 1994)
Individual evidence
- ↑ Albert Eschenmoser: Synthesis of 2,5-dimethyl-3-oxymethyl-heptadiene- (1,5) . Diploma thesis, ETH Zurich 1948, doi : 10.3929 / ethz-a-006069807 .
- ↑ Dr. Hans Schinz (1899–1990), a former doctoral student from Ruzicka, worked until his retirement as a research chemist at Firmenich Co., Geneva, at the Ruzicka Institute at ETH Zurich, where he did an internship in organic chemistry with his own doctoral students during the war supervised for students of the natural science department. See biographical information about Hans Schinz in Helv. Chim. Acta. 93 (2010), 1439, doi: 10.1002 / hlca.201000255
- ↑ a b c Albert Eschenmoser: On acid-catalyzed cyclization in mono- and sesquiterpene compounds . ETH Zurich 1952, doi : 10.3929 / ethz-a-000087828 (PhD thesis No. 2018).
- ↑ Biographies, publications and academic family tree of Albert Jakob Eschenmoser at academictree.org, accessed on February 4, 2018.
- ↑ Albert Eschenmoser: On the knowledge of acid-catalyzed cyclizations of polyene compounds of the terpene series . Habilitation thesis, ETH Zurich 1956, doi : 10.3929 / ethz-a-006078807 .
- ↑ Christian Noe on scienceblog.at
- ↑ The Krishnamurthy Lab on scripps.edu
- ↑ L. Ruzicka, A. Eschenmoser, H. Heusser: Biogenesis of Steroids and Terpenic Compounds . In: Experientia . tape 9 , 1953, pp. 362-366 . in L. Ruzicka: Biogenesis of Steroids and Terpenic Compounds . In: Experientia . tape 9 , 1953, pp. 357-367 .
- ↑ a b G. Gamboni, H. Schinz, A. Eschenmoser: About the steric course of the acid-catalyzed cyclization in the terpene series. Cyclization of cis-7-methyloctadiene (2,6) acid (1) . In: Helv. Chim. Acta . tape 37 , no. 4 , 1954, pp. 964-971 , doi : 10.1002 / hlca.19540370404 .
- ↑ a b P.A. Stadler, A. Nechvatal, AJ Frey, A. Eschenmoser: Studies on the steric course of acid-catalyzed cyclizations in terpenoid polyene compounds. Cyclization of 7,11-dimethyl-2 (trans), 6 (trans), 10-dodecatrienic and 7,11-dimethyl-2 (cis), 6 (trans), 10-dodecatrienic acid . In: Helv. Chim. Acta . tape 40 , no. 5 , 1957, pp. 1373-1409 , doi : 10.1002 / hlca.19570400527 .
- ↑ A. Eschenmoser, L. Ruzicka, O. Jeger, D. Arigoni: A stereochemical interpretation of the biogenetic isoprene rule in the triterpenes . In: Helv. Chim. Acta . tape 38 , no. 7 , 1955, pp. 1890–1904 , doi : 10.1002 / hlca.19550380728 . ; A. Eschenmoser, D. Arigoni: Revisited after 50 Years: The 'Stereochemical Interpretation of the Biogenetic Isoprene Rule for the Triterpenes' . In: Helv. Chim. Acta . tape 88 , no. 12 , 2005, p. 3011-3050 , doi : 10.1002 / hlca.200590245 .
- ↑ J. Schreiber, A. Eschenmoser: About the relative speed of the chromic acid oxidation of secondary, alicyclic alcohols . In: Helv. Chim. Acta . tape 38 , no. 6 , 1955, pp. 1529–1536 , doi : 10.1002 / hlca.19550380627 .
- ↑ L. Tenud, S. Farooq, J. Seibl, A. Eschenmoser: Endocyclic S N reactions on saturated carbon? In: Helv. Chim. Acta . tape 53 , no. 8 , 1970, pp. 2059-2069 , doi : 10.1002 / hlca.19700530816 .
- ↑ D. Felix, A. Eschenmoser: Slow Inversion at Pyramidal Nitrogen: Isolation of Diastereomeric 7-Chloro-7-azabicyclo- [4.1.0] heptanes at Room Temperature . In: Angewandte Chemie International Edition in English . tape 7 , no. 3 , 1968, p. 224-225 , doi : 10.1002 / anie.196802241 .
- ↑ K. Müller, A. Eschenmoser: Slow inversion on pyramidal bound nitrogen: Isolation and epimerization of diastereomeric N-methoxy-3,3-dimethoxycarbonyl-5-cyano-1,2-oxazolidines . In: Helv. Chim. Acta . tape 52 , no. 7 , 1969, p. 1823-1830 , doi : 10.1002 / hlca.19690520707 .
- ↑ A. Eschenmoser, A. Frey: About the cleavage of the mesyl ester of 2-methyl-2-oxymethyl-cyclopentanone with bases . In: Helv. Chim. Acta . tape 35 , no. 5 , 1952, pp. 1660–1666 , doi : 10.1002 / hlca.19520350532 .
- ↑ A. Eschenmoser, D. Felix, G. Ohloff: A novel fragmentation of cyclic α, β- unsaturated carbonyl systems; Synthesis of exaltone and rac-muscone from cyclododecanone . In: Helv. Chim. Acta . tape 50 , no. 2 , 1967, p. 708-713 , doi : 10.1002 / hlca.19670500232 . ; D. Felix, RK Müller, U. Horn, R. Joos, J. Schreiber, A. Eschenmoser: α, β- epoxy ketone → alkynone fragmentation II: pyrolytic decomposition of the hydrazones from α, β- epoxy ketones and N-amino aziridines . On synthetic methods, Part 4 . In: Helv. Chim. Acta . tape 55 , no. 4 , 1972, p. 1276-1319 , doi : 10.1002 / hlca.19720550424 .
- ↑ D. Sternbach, M. Shibuya, F. Jaisli, M. Bonetti, A. Eschenmoser: A Fragmentational Approach to Macrolides: (5-E, 8-Z) -6-methyl-5,8-undecadien-11-olide . In: Angew. Chem., Int. Ed. Engl. Volume 18 , no. 8 , 1979, pp. 634-636 , doi : 10.1002 / anie.197906341 .
- ↑ UM Kempe, TK Das Gupta, K. Blatt, P. Gygax, Dorothee Felix, A. Eschenmoser: α-Chlor-nitrone I: Representation and Ag + -induced reaction with olefins . In: Helv. Chim. Acta . tape 55 , no. 6 , 1972, p. 2187-2198 , doi : 10.1002 / hlca.19720550640 .
- ↑ EJ Corey, D. Seebach: Synthesis of 1, n-Dicarbonyl Derivates Using Carbanions from 1,3-Dithianes . In: Angew. Chem., Int. Ed. Engl. Volume 4 , no. 12 , 1965, p. 1077-1078 , doi : 10.1002 / anie.196510771 . ; D. Seebach: Methods of Reactivity Umpolung . In: Angew. Chem., Int. Ed. Engl. Volume 18 , no. 4 , 1979, p. 239-258 , doi : 10.1002 / anie.197902393 .
- ^ J. Schreiber, M. Pesaro, W. Leimgruber, A. Eschenmoser: About a new way of formation of the troponic system . In: Helv. Chim. Acta . tape 41 , no. 7 , 1958, pp. 2103-2108 , doi : 10.1002 / hlca.19580410718 . ; D. Felix, P. Jakober, A. Eschenmoser: Alkali-induced elimination of HBr in 5-bromo-β-ionone derivatives . In: Chimia . tape 19 , 1965, p. 538 .
- ↑ a b c E. Bertele, H. Boos, JD Dunitz, F. Elsinger, A. Eschenmoser, I. Felner, HP Gribi, H. Gschwend, EF Meyer, M. Pesaro, R. Scheffold: A Synthetic Route to the Corrin system . In: Angew. Chem., Int. Ed. Engl. Volume 3 , no. 7 , 1964, pp. 490-496 , doi : 10.1002 / anie.196404901 .
- ↑ a b Y. Yamada, D. Miljkovic, P. Wehrli, B. Golding, P. Löliger, R. Keese, K. Müller, A. Eschenmoser: A New Type of Corrin Synthesis . In: Angew. Chem., Int. Ed. Engl. Volume 8 , 1969, p. 343-348 , doi : 10.1002 / anie.196903431 .
- ↑ M. Roth, P. Dubs, E. Götschi, A. Eschenmoser: Sulphide contraction via alkylative coupling: a method for the preparation of β-dicarbonyl derivatives . In: Helv. Chim. Acta . tape 54 , no. 2 , 1971, p. 710-734 , doi : 10.1002 / hlca.19710540229 .
- ^ A b A. Fischli, A. Eschenmoser: A Synthetic Route to Metal-free Corrins . In: Angew. Chem., Int. Ed. Engl. Volume 6 , no. 10 , 1967, p. 866-868 , doi : 10.1002 / anie.196708661 .
- ↑ A. Wick, D. Felix, K. Steen, A. Eschenmoser: Claisen rearrangements in allyl and benzyl alcohols with the help of acetals of N, N-dimethylacetamide . In: Helv. Chim. Acta . tape 47 , no. 8 , 1964, pp. 2425-2429 , doi : 10.1002 / hlca.19640470835 .
- ↑ J. Schreiber, H. Maag, N. Hashimoto, A. Eschenmoser: Dimethyl (methylene) ammonium Iodide . In: Angew. Chem., Int. Ed. Engl. Volume 10 , no. 5 , 1971, p. 330–331 , doi : 10.1002 / anie.197103301 .
- ↑ a b R.B. Woodward: The Total Synthesis of Vitamin B 12 . In: Pure Appl. Chem. Band 33 , 1973, pp. 145-178 , doi : 10.1351 / pac197333010145 .
- ↑ Entry on Grob-Eschenmoser fragmentation. In: Römpp Online . Georg Thieme Verlag, accessed April 30, 2015.
- ↑ J. Schreiber, W. Leimgruber, M. Pesaro, P. Schudel, A. Eschenmoser: Synthesis of Colchicine . In: Angew. Chem. Band 71 , no. 20 , 1959, pp. 637-640 , doi : 10.1002 / anie.19590712002 . ; J. Schreiber, W. Leimgruber, M. Pesaro, P. Schudel, T. Threlfall, A. Eschenmoser: Synthesis of Colchicine . In: Helv. Chim. Acta. tape 44 , no. 2 , 1961, p. 540–597 , doi : 10.1002 / hlca.19610440225 .
- ^ A. Eschenmoser: Roads to Corrins (Centenary Lecture) . In: Q. Rev., Chem. Soc. tape 24 , 1970, pp. 366-415 , doi : 10.1039 / qr9702400366 . Comprehensive overview in Helv. Chim. Acta Special Issue 11/12 Dec. 2012: Albert Eschenmoser: Introductory Remarks on the Publication Series 'Corrin Syntheses-Parts I-VI' . In: Helvetica Chimica Acta . 98, No. 11-12, 2015, ISSN 0018-019X , pp. 1475-1482. doi : 10.1002 / hlca.201400399 . Albert Eschenmoser: Corrin Syntheses. Part I . In: Helvetica Chimica Acta . 98, No. 11-12, 2015, ISSN 0018-019X , pp. 1483-1600. doi : 10.1002 / hlca.201400277 .
- ↑ A partial synthesis of vitamin B 12 from cobyric acid had already been carried out in 1960: W. Friedrich, G. Gross, K. Bernhauer, P. Zeller: Syntheses in the vitamin B12 area. 4. Communication partial synthesis of vitamin B12 . In: Helvetica Chimica Acta . tape 43 , 1960, pp. 704-712 , doi : 10.1002 / hlca.19600430314 .
- ↑ Walter Fuhrer: Total synthesis of vitamin B-12: the photochemical way . ETH Zurich 1973, doi : 10.3929 / ethz-a-000086601 (doctoral thesis No. 5158). ; Hans Maag: Total synthesis of vitamin B-12: Dicyano-Co (III) -Cobyrinsäure-Hexamethylester-f-amide . ETH Zurich 1973, doi : 10.3929 / ethz-a-000085446 (PhD thesis No. 5173). ; Peter Schneider: Total synthesis of derivatives of Dicyano-cobalt (III) -5,15-bis-nor-cobyrinic acid hepta-methyl ester . ETH Zurich 1972, doi : 10.3929 / ethz-a-000090603 (PhD thesis No. 4819).
- ↑ a b c A. Eschenmoser: Studies on Organic Synthesis . In: 23rd Int. Congress of Pure and Applied Chemistry, Boston, Pure & Appl. Chem. Supplement Vol. 2, 1971, pp. 69-106 , doi : 10.3929 / ethz-a-010165162 (English). ; A. Eschenmoser, "The Final Phase of the Harvard / ETH Collaboration on the Synthesis of Vitamin B12", in: Albert Eschenmoser: Corrin Syntheses. Part I . In: Helvetica Chimica Acta . 98, No. 11-12, 2015, ISSN 0018-019X , pp. 1483-1600. doi : 10.1002 / hlca.201400277 . , ch. 3 (pp. 1555-1574)
- ↑ A. Eschenmoser, CE Wintner: Natural Product Synthesis and Vitamin B12 . In: Science (Washington, DC, US) . tape 196 , no. 4297 , 1977, pp. 1410-1420 , doi : 10.1126 / science.867037 .
- ↑ a b R.B. Woodward: Revent Advances in the Chemistry of Natural Products . In: Pure Appl. Chem. Band 17 , 1968, p. 519-547 , doi : 10.1351 / pac196817030519 .
- ^ RB Woodward: Recent Advances in the Chemistry of Natural Products . In: Pure Appl. Chem. Band 25 , 1971, p. 283-304 , doi : 10.1351 / pac197125010283 .
- ^ A. Eschenmoser: Epilogue: Synthesis of Coenzyme B12: A Vehicle for the Teaching of Organic Chemistry . In: G. Quinkert, MV Kisakürek (Ed.): Essays in Contemporary Chemistry: From Molecular Structure towards Biology . Verlag Helvetica Chimica Acta, Weinheim 2001, p. 391-441 , doi : 10.1002 / 9783906390451.ch12 .
- ↑ a b A.R. Battersby, E. McDonald: Biosynthesis of Porphyrins, Chlorins and Corrins . In: KM Smith (Ed.): Porphyrins and Metalloporphyrins . Elsevier Science Publishers, Amsterdam 1975, ISBN 978-0-444-41375-8 , pp. 61-122 . ; AI Scott: Tetrahedron report: Concerning the biosynthesis of vitamin B 12 . In: Tetrahedron . tape 31 , no. 21 , 1975, p. 2639-2653 , doi : 10.1016 / 0040-4020 (75) 80326-7 .
- ↑ A. Pfaltz, N. Bühler, R. Neier, K. Hirai, A. Eschenmoser: Photochemical and non-photochemical A / D-Secocorrin → Corrin cyclizations in 19-carboxy- and 19-formyl-1-methylidene-1 , 19-secocorrinaten. Decarboxylability and deformylability of nickel (II) -19-carboxy- and 19-formyl-corrinates . In: Helv. Chim. Acta 60 . tape 60 , no. 8 , 1977, pp. 2653–2672 , doi : 10.1002 / hlca.19770600817 .
- ↑ a b A. Eschenmoser: Post-B12 Problems in Corrin Synthesis (Robert Robinson Lecture) . In: Chem. Soc. Rev. Band 5 , 1976, p. 377-410 , doi : 10.1039 / cs9760500377 .
- ^ AP Johnson, P. Wehrli, R. Fletcher, A. Eschenmoser: Corphin, a Corrinoid-Porphinoid Ligand System . In: Angew. Chem., Int. Ed. Engl. Volume 7 , no. 8 , 1968, p. 623-625 , doi : 10.1002 / anie.196806231 . ; PM Müller, S. Farooq, B. Hardegger, WS Salmond, A. Eschenmoser: Metal-Free Derivatives of the Corphin Ligand System . In: Angew. Chem., Int. Ed. Engl. Volume 12 , no. 11 , 1973, p. 914-916 , doi : 10.1002 / anie.197309141 .
- ^ A b A. Eschenmoser: Chemistry of Corphinoids . In: Ann. NY Acad. Sci. tape 471 , 1986, pp. 108-129 , doi : 10.1111 / j.1749-6632.1986.tb48030.x .
- ↑ entry to corphin (e). In: Römpp Online . Georg Thieme Verlag, accessed April 30, 2015.
- ↑ a b R. Waditschatka, A. Eschenmoser: The Chemistry of Pyrrocorphins: Stereoselectivity in the Porphyrinogen → Pyrrocorphin Tautomerization . In: Angew. Chem., Int. Ed. Engl. Volume 22 , no. 8 , 1983, p. 630-631 , doi : 10.1002 / anie.198306301 .
- ↑ R. Schwesinger, R. Waditschatka, J. Rigby, R. Nordmann, WB Schweizer, E. Zass, A. Eschenmoser: The pyrrocorphine ligand system: synthesis of 2,2,7,7,12,12,17-heptamethyl -2,3,7,8,12,13-hexahydroporphyrins . In: Helv. Chim. Acta . tape 65 , no. 2 , 1982, p. 600–610 , doi : 10.1002 / hlca.19820650220 .
- ↑ A. Pfaltz, B. Jaun, A. Fässler, A. Eschenmoser; R. Jaenchen, HH Gilles, G. Diekert, RK Thauer: To the knowledge of the factor F430 from methanogenic bacteria: Structure of the porphinoid ligand system . In: Helv. Chim. Acta . tape 65 , no. 3 , 1982, pp. 828-865 , doi : 10.1002 / hlca.19820650320 .
- ↑ A. Fässler, A. Pfaltz, B. Kräutler, A. Eschenmoser: Chemistry of Corphinoids: Synthesis of a Nickel (II) Complex Containing the Chromophore System of Coenzyme F430 . In: J. Chem. Soc., Chem. Commun. No. 20 , 1984, pp. 1365-1367 , doi : 10.1039 / C39840001365 .
- ↑ a b A. Eschenmoser: Vitamin B12: Experiments Concerning the Origin of its Molecular Structure . In: Angew. Chem., Int. Ed. Engl. Volume 27 , no. 1 , 1988, p. 5-39 , doi : 10.1002 / anie.198800051 .
- ↑ a b c A. Eschenmoser: Etiology of Potentially Primordial Biomolecular Structures: From Vitamin B12 to the Nucleic Acids and an Inquiry into the Chemistry of Life's Origin: a Retrospective . In: Angew. Chem., Int. Ed. tape 50 , no. 52 , 2011, p. 12412-12472 , doi : 10.1002 / anie.201103672 .
- ^ V. Rasetti, A. Pfaltz, C. Kratky, A. Eschenmoser: Ring Contraction of Hydroporphinoid to Corrinoid Complexes . In: Proc. Natl. Acad. Sci. USA band 78 , no. 1 , 1981, p. 16-19 , doi : 10.1073 / pnas.78.1.16 . ; V. Rasetti, K. Hilpert, A. Fässler, A. Pfaltz, A. Eschenmoser: The Dihydrocorphinol → Corrin Ring Contraction: A Potentially Biomimetic Mode of Formation of the Corrin Structure . tape 20 , no. 12 , 1981, p. 1058-1060 , doi : 10.1002 / anie.198110581 .
- ↑ C. Kratky; R. Waditschatka, C. Angst, JE Johansen, JC Plaquevent, J. Schreiber, A. Eschenmoser: The saddle conformation of the hydroporphinoid nickel (II) complexes: structure, origin and stereochemical consequences . In: Helv. Chim. Acta . tape 68 , no. 5 , 1985, pp. 1312-1337 , doi : 10.1002 / hlca.19850680526 .
- ↑ C. Kratky, A. Fässler, A. Pfaltz, B. Kräutler, B. Jaun, A. Eschenmoser: Chemistry of Corphinoids: Structural Properties of Corphinoid Nickel (II) Complexes related to Coenzme F430 . In: J. Chem. Soc., Chem. Commun. No. 20 , 1984, pp. 1368-1371 , doi : 10.1039 / C39840001368 .
- ↑ Kaspar Zimmermann: Comparative studies on cobaltcorphine and cobaltcorrine complexes . ETH Zurich 1989, doi : 10.3929 / ethz-a-000541314 (PhD thesis No. 9038).
- ↑ R. Waditschatka, E. Diener, A. Eschenmoser: The Chemistry of Pyrrocorphins: C-methylation of Pyrrocorphinates at the ligand periphery . In: Angew. Chem., Int. Ed. Engl. Volume 22 , no. 8 , 1983, p. 631-632 , doi : 10.1002 / anie.198306311 . ; C. Leumann, K. Hilpert, J. Schreiber, A. Eschenmoser: Chemistry of Pyrrocorphins: C-Methylations at the Periphery of Pyrrocorphins and Related Corphinoid Ligand Systems . In: J. Chem. Soc., Chem. Commun. No. 23 , 1983, pp. 1404-1407 , doi : 10.1039 / C39830001404 .
- ↑ Fritz Kreppelt: Regioselective reconstitution of vitamin B12 by Nukleotidierung of Cobyrinsaure-heptakis (cyanomethyl) ester . ETH Zurich 1991, doi : 10.3929 / ethz-a-000626280 (doctoral thesis No. 9458).
- ↑ a b J. Hunziker, H.-J. Roth, M. Böhringer, A. Giger, U. Diederichsen, M. Göbel, R. Krishnan, B. Jaun, C. Leumann, A. Eschenmoser: Why pentose and not hexose nucleic acids? Part III. Oligo (2 ', 3'-dideoxy-β-D-glucopyranosyl) nucleotide (' homo-DNA '): pairing properties . In: Helv. Chim. Acta . tape 76 , 1993, pp. 259–352 , doi : 10.1002 / hlca.19930760119 .
- ↑ A. Eschenmoser: Chemical Etiology of Nucleic Acid Structure . In: Science (Washington, DC, US) . tape 284 , no. 5423 , 1999, p. 2118-2124 , doi : 10.1126 / science.284.5423.2118 . ; A. Eschenmoser: Why pentose and not hexose nucleic acids? In: Nachr. Chem., Tech. Lab. tape 39 , no. 7-8 , 1991, pp. 795-807 , doi : 10.1002 / nadc.19910390707 .
- ^ S. Pitsch, S. Wendeborn, B. Jaun, A. Eschenmoser: Why Pentose- and not Hexose- Nucleic Acids? Pyranosyl RNA ("p-RNA") . In: Helv. Chim. Acta . tape 76 , no. 6 , 1993, pp. 2161-2183 , doi : 10.1002 / hlca.19930760602 . ; M. Beier, F. Reck, T. Wagner, R. Krishnamurthy, A. Eschenmoser: Chemical Etiology of Nucleic Acid Structure: Comparing Pentopyranosyl- (2 '& rarr4') Oligonucleotides with RNA . In: Science (Washington, DC, US) . tape 283 , no. 5402 , 1999, pp. 699-703 , doi : 10.1126 / science.283.5402.699 .
- ↑ a b K.-U. Schöning, P. Scholz, S. Guntha, X. Wu, R. Krishnamurthy, A. Eschenmoser: Chemical Etiology of Nucleic Acid Structure: The α-Threofuranosyl- (3 ' → 2') Oligonucleotide System . In: Science (Washington, DC, US) . tape 290 , no. 5495 , 2000, pp. 1347-1351 , doi : 10.1126 / science.290.5495.1347 . ; K.-U. Schöning, P. Scholz, X.Wu, S. Guntha, G. Delgado, R. Krishnamurthy, A. Eschenmoser: The α-L-Threofuranosyl- (3'-2 ') - oligonucleotide system (' TNA '): Synthesis and pairing properties . In: Helv. Chim. Acta . tape 85 , no. 12 , 2002, p. 4111-4153 , doi : 10.1002 / hlca.200290000 .
- ^ I. Schlönvogt, S. Pitsch, C. Lesueur, A. Eschenmoser, B. Jaun, RM Wolf: Pyranosyl-RNA ('p-RNA'): NMR and Molecular-Dynamics Study of the Duplex Formed by Self-pairing of Ribopyranosyl- (CGAATTCG) . In: Helv. Chim. Acta . tape 79 , no. 8 , 1996, pp. 2316–2345 , doi : 10.1002 / hlca.19960790820 .
- ↑ M.-O. Ebert, C. Mang, R. Krishnamurthy, A. Eschenmoser, B. Jaun: The Structure of a TNA-TNA Complex in Solution: NMR Study of the Octamer Duplex Derived from α- (L) -Threofuranosyl- (3 ' → 2 ') -CGAATTCG . In: J. Am. Chem. Soc. tape 130 , 2008, p. 15105-15115 , doi : 10.1021 / ja8041959 .
- ↑ M. Bolli, R. Micura, A. Eschenmoser: Pyranosyl-RNA: Chiroselective Self-assembly of Base Sequences by Ligative Oligomerization of Tetranucleotide-2 ', 3'-cyclophosphates (with a Commentary Concerning the Origin of Biomolecular Homochirality) . In: Chem. Biol. (Oxford, UK) . tape 4 , no. 4 , 1997, p. 309-320 , doi : 10.1016 / S1074-5521 (97) 90074-0 .
- ↑ G. Ksander, G. Bold, R. Lattmann, C. Lehmann, T. Früh, Y.-B. Xiang, K. Inomata, H.-P. Buser, J. Schreiber, E. Zass, A. Eschenmoser: Chemistry of α-aminonitriles. 1st communication: Introduction and ways to uroporphyrinogenoctanitriles . In: Helv. Chim. Acta . tape 70 , no. 4 , 1987, pp. 1115–1172 , doi : 10.1002 / hlca.19870700424 .
- ↑ S. Drenkard, J. Ferris, A. Eschenmoser: Chemistry of α-aminonitriles. Aziridine-2-carbonitrile: photochemical formation from 2-aminopropenenitrile . In: Helv. Chim. Acta . tape 73 , no. 5 , 1990, pp. 1373-1390 , doi : 10.1002 / hlca.19900730524 .
- ^ R. Krishnamurthy, G. Arrhenius, A. Eschenmoser: Formation of Glycolaldehyde Phosphate from Glycolaldehyde in Aqueous Solution . In: Origins Life Evol. Biospheres . tape 29 , 1999, pp. 333 . ; R. Krishnamurthy, S. Guntha, A. Eschenmoser: Regioselective α-Phosphorylation of Aldoses in Aqueous Solution . In: Angew. Chem., Int. Ed. tape 39 , 2000, pp. 2281-2285 , doi : 10.1002 / 1521-3773 (20000703) 39:13 <2281 :: AID-ANIE2281> 3.0.CO; 2-2 .
- ↑ D. Müller, S. Pitsch, A. Kittaka, E. Wagner, CE Wintner, A. Eschenmoser: Chemistry of α-aminonitriles. Aldomerization of glycolaldehyde phosphate to racemic hexose-2,4,6-triphosphates and (in the presence of formaldehyde) racemic pentose-2,4-diphosphates: rac-allose-2,4,6-triphosphate and rac-ribose-2, 4-diphosphate are the main products of the reaction . In: Helv. Chim. Acta . tape 73 , no. 5 , 1990, pp. 1410–1468 , doi : 10.1002 / hlca.19900730526 .
- ^ A. Eschenmoser: The Search for the Chemistry of Life's Origin . In: Tetrahedron . tape 63 , no. 52 , 2007, p. 12821-12844 , doi : 10.1016 / j.tet.2007.10.012 .
- ↑ A. Eschenmoser: On Organic Natural Product Synthesis and Vitamin B12 (RA Welch Award Address) . In: Proc. Robert A. Welch Found. Conf. Chem. Res. Volume 18 , 1974, p. 269 .
- ↑ A. Eschenmoser: Questioning natural product structures . In: Helv. Chim. Acta . tape 93 , no. 8 , 2010, p. 1439–1499 , doi : 10.1002 / hlca.201000255 .
literature
The scientific work of Albert Eschenmoser is unusually broad in terms of content and comprises over 270 publications: Albert Eschenmoser List of Scientific Publications . In: Heterocycles . tape 82 , 2010, p. 31 , doi : 10.3987 / COM-10-S (E) publications .
Literature about Albert Eschenmoser:
- "Mr. Woodward regrets that the matter is finished": Woodward and Eschenmoser on vitamin B 12 and the situation of organic chemistry (interview) . In: News from chemistry and technology . tape 20 , 1972, p. 147-150 .
- D. Arigoni: Professor Albert Eschenmoser on his 60th birthday . In: Chimia . tape 39 , 1985, pp. 336 .
- WR Pötsch, A. Fischer, W. Müller, H.Cassebaum: Lexicon of important chemists . Verlag Harri Deutsch, Thun 1989, ISBN 3-8171-1055-3 , p. 140 .
- V. Prelog: Albert Eschenmoser . In: Aldrichimica Acta . tape 23 , 1990, pp. 59-64 .
- E. Zass: Homage to Albert Eschenmoser . In: Chimia . tape 47 , 1993, pp. 154-159 .
- EJ Sorensen: Albert Eschenmoser . In: Helv. Chim. Acta . tape 83 , 2000, pp. 1673-1677 , doi : 10.1002 / 1522-2675 (20000809) 83: 8 <1673 :: AID-HLCA1673> 3.0.CO; 2-I .
- G. Quinkert: Preface . In: G. Quinkert, MV Kisakurek (Ed.): Hominatio: An International Tribute to Albert Eschenmoser . Verlag Helvetica Chimica Acta, Zurich 2001, ISBN 978-3-906390-27-7 , VII-XIII, doi: 10.1002 / 9783906390451.fmatter
- I. Hargittai: Albert Eschenmoser (interview). In: M. Hargittai (Ed.): Candid Science III: More Conversations with Famous Chemists . Imperial College Press, London 2003, ISBN 978-1-86094-336-2 , pp. 79-108, doi : 10.1142 / 9781848161344_0007
- N. Hall: The quest for the chemical roots of life . In: Chemical Communications (Cambridge, United Kingdom) . 2004, p. 1247-1252 , doi : 10.1039 / b401124b .
- Curriculum vitae Albert Eschenmoser . tape 82 , 2010, p. 15-23 , doi : 10.3987 / COM-10-S (E) CV .
- B. Kräutler: Congratulations To Professor Albert Eschenmoser On His 85th Birthday . In: Heterocycles . tape 82 , 2010, p. 1-4 , doi : 10.3987 / COM-10-S (E) Foreword_1 .
- SE Denmark, EJ Sorensen: Preface To Special Issue Of Heterocycles Honoring The 85th Birthday Of Prof. Dr. Albert Eschenmoser . In: Heterocycles . tape 82 , 2010, p. 5-10 , doi : 10.3987 / COM-10-S (E) Foreword_2 .
- L. Weber: "Successful moments in research are extremely pleasurable!" Interview with Albert Eschenmoser, new honorary member of the SCG . In: Chimia . tape 64 , 2010, p. 323-327 , doi : 10.2533 / chimia.2010.323 .
Web links
- Engelbert Zass: Eschenmoser, Albert. In: Historical Lexicon of Switzerland . November 21, 2005 , accessed October 27, 2019 .
- Prof. Dr. Albert Eschenmoser (CV, ETH Zurich , Department of Chemistry and Applied Biosciences)
- Literature by and about Albert Eschenmoser in the catalog of the German National Library
- On the aetiology of the structure type of natural nucleic acids ( Rudolf Criegee lecture 2005, University of Karlsruhe )
- Michael Marshall: The secret of how life on earth began , on: BBC - Earth, October 31, 2016
| personal data | |
|---|---|
| SURNAME | Eschenmoser, Albert |
| ALTERNATIVE NAMES | Eschenmoser, Albert Jakob |
| BRIEF DESCRIPTION | Swiss chemist |
| DATE OF BIRTH | August 5, 1925 |
| PLACE OF BIRTH | Erstfeld , Switzerland |