Polarity reversal
In organic chemistry, polarity reversal is the name given to the modification of a functional group with the aim of reversing its polarity. This also reverses their reactivity: an electron deficiency can lead to an excess of electrons; there are new ways to react. The Germanismus umpolung is also established in the English-speaking world.
Many organic reactions proceed via polar donor and acceptor centers, with negatively polarized atoms in the molecule joining as nucleophiles with positively polarized atoms (or electrophilic centers).
Due to their higher electronegativity, heteroatoms such as oxygen or nitrogen in a carbon structure cause a higher electron density on the heteroatom and a positive polarization on the adjacent carbon atom . This creates alternating donor and acceptor centers on a carbon skeleton, which lead to a normal reaction pattern in polar reactions.
The polarity reversal method is often used to establish a CC link. The principle here is to generate a negatively polarized carbon atom, which can then attack a positively polarized carbon atom nucleophilically .
Dieter Seebach and EJ Corey introduced the concept. Classic examples of polarity reversal are the Grignard reaction and the benzoin condensation .
Carbonyl Umpolung
A well-known example is the Dithian method or Corey-Seebach reaction . For example, a carbonyl group of an aldehyde is converted into a thioacetal, the previous carbonyl carbon is after deprotonation with z. B. n -Butyllithium to the nucleophile, which reacts with haloalkanes, carbonyl components or oxiranes in a nucleophilic substitution. After hydrolysis of the thioacetal, z. B. α-alkyl ketones.
Polarity reversal by forming a Grignard connection
Umpolung takes place in the synthesis of Grignard compounds from haloalkanes .
Umpolung of the methylene group of diethyl malonate on reaction with ethanolate
The methylene group of the diethyl malonate is strongly positively polarized by the two adjacent carboxyl groups and depleted of electrons. A proton is split off by the strong base ethanolate and the polarity of the carbon atom is reversed. This carbon atom can be alkylated by reaction with alkyl or aryl halides, see malonic ester synthesis .
Polarity reversal catalysts
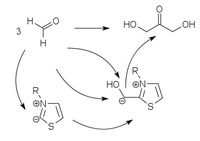
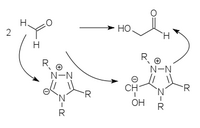
Strongly acidic CH compounds (cyanide, thiazolium or triazolium heterocycles) can add to carbonyl compounds as carbanions and thereby make their CH bonds so acidic that the carbanions formed from them can be reacted as nucleophiles. This corresponds to a polarity reversal of the electrophilic carbonyl C atom to the nucleophilic carbanion. The CC bond to the acidic catalyst is very labile and easily dissolves, so that the catalyst is available again for a new catalytic cycle.
The classic example of such a umpolung catalysis is the benzoin condensation with cyanide, which only works with aromatic aldehydes. Heterocyclic umpolung catalysts (thiazolium or triazolium heterocycles) can also carry out this condensation with aliphatic aldehydes. Thiazolium ylides , triazolium ylides and perimidinium ylides
| Catalytic polarity reversal | ||
|
|

|
| Thiazolium catalysis | Triazolium catalysis | Perimidinium catalysis |
See also
Web links
Individual evidence
- ^ Seebach D .: Methods of Reactivity Umpolung . In: Angewandte Chemie International Edition in English . 18, No. 4, 1979, pp. 239-258. doi : 10.1002 / anie.197902393 .
- ↑ Gröbel BT, Seebach D .: Methods of Reactivity Umpolung Reactivity of Carbonyl Compounds Through Sulfur-Containing Reagents . In: Synthesis . 6, 1977, pp. 357-402. doi : 10.1055 / s-1977-24412 .
- ↑ Seebach D., Corey EJ: Generation and synthetic applications of 2-lithio-1,3-dithianes . In: Journal of Organic Chemistry . 40, No. 2, 1975, pp. 231-237. doi : 10.1021 / jo00890a018 .
- ↑ DE000004122669A1 (1991) Process for the production of dihydroxyacetone.
- ↑ DE000004212264A1 (1992) Process for the catalytic production of condensation products of formaldehyde.
- ↑ DE000004230466A1 (1992) Process for the catalytic production of condensation products of formaldehyde.
- ↑ DE000019536403A1 (1995) Perimidinium salts, their production and use.


