Carboxy group

The carboxy group (older, still used term carboxyl group , also carboxylic acid group ) is the functional group –COOH of carboxylic acids in chemistry . The name is formally derived from the combination of the two contained elements carbonyl group and hydroxyl group .
The carboxy carbon atom carries a double bonded oxygen atom and a single bonded hydroxyl group. Due to the electronegativity difference between oxygen and carbon, the carbon atom carries a partial positive charge . The carboxyl carbon atom is therefore easily vulnerable to nucleophilic attack. The hydroxyl unit of the carboxy group is relatively acidic, the proton is easily given off to an appropriate partner. This increased in comparison to alcohols acidity (acid strength) is located in the resonance stabilization of the corresponding base, the negatively charged carboxylate - anion justified. In the carboxylate anion, the oxygen atoms are equivalent, i.e. the negative charge is distributed over both oxygen atoms and both CO bonds have partial double bond character.
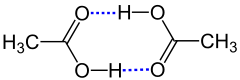
In solution and crystal, carboxylic acids are often present as dimers , with the carbonyl and hydroxyl oxygen of two different molecules being hydrogen-bonded by the hydroxy proton . The pair formation allows the formation of two hydrogen bonds per dimer.
As a component of carboxylic and amino acids, carboxy groups are one of the most common functional groups found in nature.
Web links
Individual evidence
- ↑ Entry on carboxyl group. In: Römpp Online . Georg Thieme Verlag, accessed on August 28, 2019.
- ↑ Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 1: A-Cl. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1979, ISBN 3-440-04511-0 , p. 601.