Retrosynthesis
The retrosynthetic analysis or retrosynthetic analysis is a technique when scheduling a chemical synthesis of complex organic molecules . In this process, the molecule is broken down into simpler building blocks, for which synthesis examples are known. In this way, you can gradually get to the building blocks that are commercially available or known from the literature. This leads to a schema that branches down like a tree. EJ Corey introduced this formalism and was awarded the Nobel Prize in Chemistry in 1990 for this work . The power of retrosynthesis shows when a new synthesis route is to be designed. The aim is to keep simplifying the desired structure. As a rule, there are several possible routes that together make up the synthesis tree. It is now the chemist's task to choose the ideal route, but it does not always have to be the shortest.
Definitions
For these formal cuts, Corey has developed a simple building block model, which can even deliver the retrosynthesis scheme via a computer program (LHASA) with the help of a reaction database. For this purpose, several possible reaction steps were abstracted and formulated in the following definitions.
- Disconnections: A retro synthetic step, the breakage of one or more bonds to a plurality of synthon means
- Retron: The starter molecule at the bottom of the retrosynthesis tree
- Retrosynthetic tree: Retrosynthetic tree
- Synthon: See own article Synthon
- Target: The target molecule
- Transform: The reverse step as in the actual synthesis operation, which may also be steps that activate a functional group involved (functional group transformation)
example
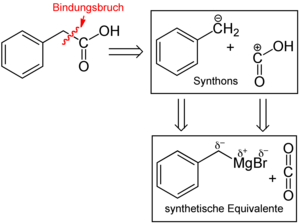
The concept of retrosynthesis can be illustrated using the simple example of phenylacetic acid :
When planning the synthesis, two synthons can be recognized. One synthon is the carboxy group - COOH as a nucleophilic synthon. The PhCH 2 + group, i.e. the benzyl cation, can be recognized as a complementary electrophilic synthon. Both synthons are not stable as a connection. For this purpose, synthetic equivalents are now being sought. One equivalent for the - COOH is the cyanide anion, the other, for the benzyl cation, would be benzyl bromide . It is characteristic of a synthetic equivalent that it reflects the electronic properties of the synthon, i.e. should have its reactivity, and that the molecule obtained by linking the synthons is chemically later by manipulating the functional group (here the nitrile group) in the target, i.e. phenylacetic acid, can be converted. In this example, the conversion would be possible through acidic or basic hydrolysis:
Alternatively , retrosynthetic analysis for the production of phenylacetic acid could also reveal two other synthons, the PhCH 2 - group and + COOH. These two synthons are also not stable as a connection. Therefore, synthetic equivalents are now being sought. An equivalent to the PhCH 2 - group is benzylmagnesium bromide (PhCH 2 MgBr) with the negatively polarized benzylic carbon atom, the synthetic equivalent to + COOH is carbon dioxide, CO 2 :
Linking these two synthons would result in PhCH 2 CO 2 MgBr, the hydrolysis of which then yields the target molecule phenylacetic acid.
Strategies
- Functional group strategy: changes in the functional group lead to a reduction in the complexity of the molecule.
- Stereochemical strategy: Many targets are stereochemically complex molecules. Stereochemical transformations can be transferred or changed through stereospecific reactions. Examples of this would be the Diels-Alder reaction , the Claisen rearrangement or the Mitsunobu reaction . This allows the target to be simplified stereochemically.
- Structure-target strategy: This bidirectional strategy approaches the target molecule from both sides, i.e. from the retrosynthetic as well as from the synthetic side, to a common intermediate. This strategy can simplify the selection or the finding of a suitable synthesis route.
- Transformation strategy: The transformations can significantly reduce the complexity of a molecule. Unfortunately, good retrons are rare and this can mean that you have to plan additional synthesis steps that activate the retron.
- Topological strategy: Finding one or more strategic cuts can lead to a key intermediate in which a rearrangement transformation of the framework is easy to recognize. As a rule, ring structures are preferably formed and that ring sizes with more than seven atoms are no longer preferred.
LHASA
LHASA (Logic and Heuristic Applied to Synthesis Analysis) is currently being developed by three working groups. LHASA calculates synthetic trees exclusively on a retrosynthetic basis. It currently has a database of 2000 transformations. The transformations are assessed using synthesis strategies developed by Corey.
- Intermediate transform ('Subgoal Transform'): LHASA is limited to transforming functional groups.
- Strategic Transform ('Goal Transform'): LHASA tries to achieve the retron of a strategic transform as the goal of each retrosynthesis step. This leads to a simplification of the synthesis goal and usually means breaking one or more bonds.
- Tactical combination ('Tactical Combination'): There is a fixed sequence of strategic transformations or intermediate-step transformations that should simplify the synthesis goal.
- Far-reaching transform ('Long-range Transform'): With this strategy LHASA tries to apply transformations that are as complex as possible, which should lead to a maximum simplification of the synthesis intermediate.
Individual evidence
- ^ EJ Corey, XM. Cheng: " The Logic of Chemical Synthesis " Wiley New York 1995 ISBN 0-471-11594-0 .
- ^ Nobel Prize Committee .
- ↑ Nobel Prize Lecture (PDF; 779 kB).
- ^ EJ Corey: "Retrosynthetic Thinking - Essentials and Examples". In Chem. Soc. Rev. 1988 , 17 , 111-133. doi : 10.1039 / CS9881700111 .
Web links
- Retrosynthesis at the networked course at Erlangen University
- Center for Molecular and Biomolecular Informatics
- Presentation on ARChem Route Designer, ACS, Philadelphia, September 2008