Synthon
A synthon is a conceptual building block in retrosynthesis planning. The underlying concept was developed by EJ Corey .
Derivation
A synthon represents a structural unit within a molecule to which a synthetic step can be assigned. The reactive sites of the synthon are denoted by the letters a for acceptor or d for donor .
example
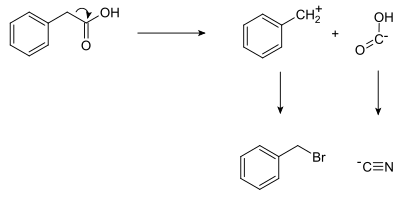
When planning the synthesis of phenylacetic acid , two synthons can be identified by theoretical division of the target molecule at the PhCH 2 - / - COOH bond: a nucleophilic “-COOH” group and an electrophilic “PhCH 2 + ” group. The two synthons do not necessarily have to exist as real molecule fragments, but the synthetic equivalents of these synthons can be used in the reaction to get to the target molecule. In this case the cyanide anion is the - COOH synthon while benzyl bromide is the synthetic equivalent for the benzyl synthon.
The synthesis route to phenylacetic acid is determined by the retrosynthesis as follows:
Examples of synthons
- : , (d-Synthon)
- : , (d-Synthon)
- Carboxylic acid : (d-Synthon, processing with )
- C1 synthons - carbon dioxide , carbon monoxide , cyanide
- C2 synthons - acetylene , acetaldehyde
- - synthons - ethylene oxide
- Carbocation synthons - alkyl halide
- Carbanion Synthons - Grignard Reagent , Organolithium Compound, Substituted Acetylides
Individual evidence
- ↑ EJ Corey: General methods for the construction of complex molecules . In: Pure and Applied Chemistry . tape 14 , 1967, p. 19-37 , doi : 10.1351 / pac196714010019 .