Corey-Seebach reaction
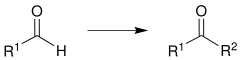
The Corey-Seebach reaction - named after Elias James Corey Jr. and Dieter Seebach - is a name reaction from the field of organic chemistry . Here are aldehydes or ketones of formaldehyde synthesized or other aldehydes. As a special feature, a temporary umpolung of a carbonyl group takes place.
Overview
An aldehyde (R 1 = organic radical) is successively reacted with propane -1,3-di thiol , deprotonated with n - butyllithium and alkylated with an electrophile (e.g. a haloalkane ). Ultimately, the Corey-Seebach reaction usually serves to synthesize a ketone (R 1 , R 2 = organic residues):
With an appropriate choice of the radicals R 1 and R 2 , this reaction can alternatively convert formaldehyde (R 1 = H) to another aldehyde (R 1 = H; R 2 = organic radical).
mechanism
Proposal for the reaction mechanism for the synthesis of a ketone 5 from an aldehyde 1 :
The aldehyde 1 is reacted with propane-1,3-dithiol and a Lewis acid to give a cyclic di thioacetal 2 . This changes the acidity of the hydrogen atom bonded to the carbon; this can now be split off with the strong base n - butyllithium . The resulting carbanion 3 is a strong nucleophile and reacts with a chloroalkane (electrophile) to form the dithioacetal 4 . In order to regain the carbonyl group, the dithioacetal 4 is reacted with mercury (II) chloride , calcium carbonate and aqueous acetone solution to give ketone 5 . The 1,3-propane-dithiol is split off again.
Practical meaning
The Corey-Seebach reaction is a purely laboratory process and has no industrial significance. Because of the formation of stoichiometric amounts of several waste materials , the atom economy of this reaction is so bad that an industrial synthesis based on this reaction is not economical. In addition, there is the stoichiometric use of toxic mercury (II) chloride in the third reaction step (cleavage of the dithioacetal).
variants
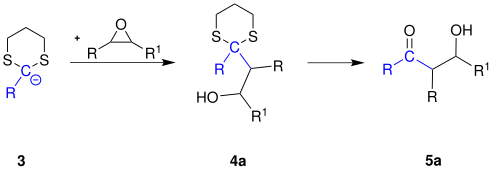
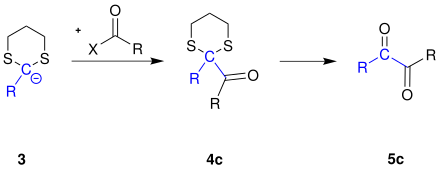
If the carbanion 3 is reacted with other electrophiles instead of a haloalkane, other products can also be obtained according to the principle of the Corey-Seebach reaction.
Examples:
The carbanion 3 is converted with an oxirane to a β - hydroxyketone 5a :
The carbanion 3 is reacted with a ketone to form an α- hydroxyketone 5b :
The carbanion 3 is reacted with a carboxylic acid halide to form a 1,2- diketone 5c :
The reaction mechanism for these reactions is analogous to the mechanism for the reaction with haloalkanes (see above).
literature
- Reinhard Brückner: reaction mechanisms . 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , pp. 384–385.
Individual evidence
- ↑ Reinhard Brückner : Reaction Mechanisms , Spectrum Akademischer Verlag, 3rd corrected edition, 2007, pp. 384–385, ISBN 978-3-8274-1579-0 .
- ↑ a b c d e Portal for Organic Chemistry: Contribution to the Corey-Seebach reaction, accessed on July 26, 2013 .
- ↑ Bradford P. Mundy, Michael G. Ellert, Frank G. Favaloro, Jr .: Name Reactions in Organic Synthesis , Wiley & Sons, 2nd Edition, 2005, pp. 186-187, ISBN 0-471-22854-0 .