Malonic ester synthesis
The Malonestersynthese is a reaction in the organic chemistry for the production of alkylated malonic acid esters . It is used by saponification and subsequent decarboxylation , substituted acetic acids to obtain. It is also possible to alkylate twice and, in the case of double substitution with the same radical, obtain symmetrically disubstituted acetic acids, but you can also introduce different substituents in two steps and then obtain asymmetrical acetic acid derivatives.
Alkylated malonic esters also serve as synthetic building blocks, e.g. B. for the production of barbituric acid derivatives such as veronal , which are used as pain relievers and sleeping pills - but are now mostly used for euthanizing animals.
mechanism
In the first step, the malonic ester is replaced by a strong base such as B. an alcoholate in the α position d. H. deprotonated on the methylene group. A haloalkane is then attacked by the deprotonated α- carbon , nucleophilically . An α-alkylated malonic ester is obtained. The ester groups are hydrolyzed with base catalysis . The previously formed dicarboxylic acid is decarboxylated by the action of heat ( CO 2 is split off) .
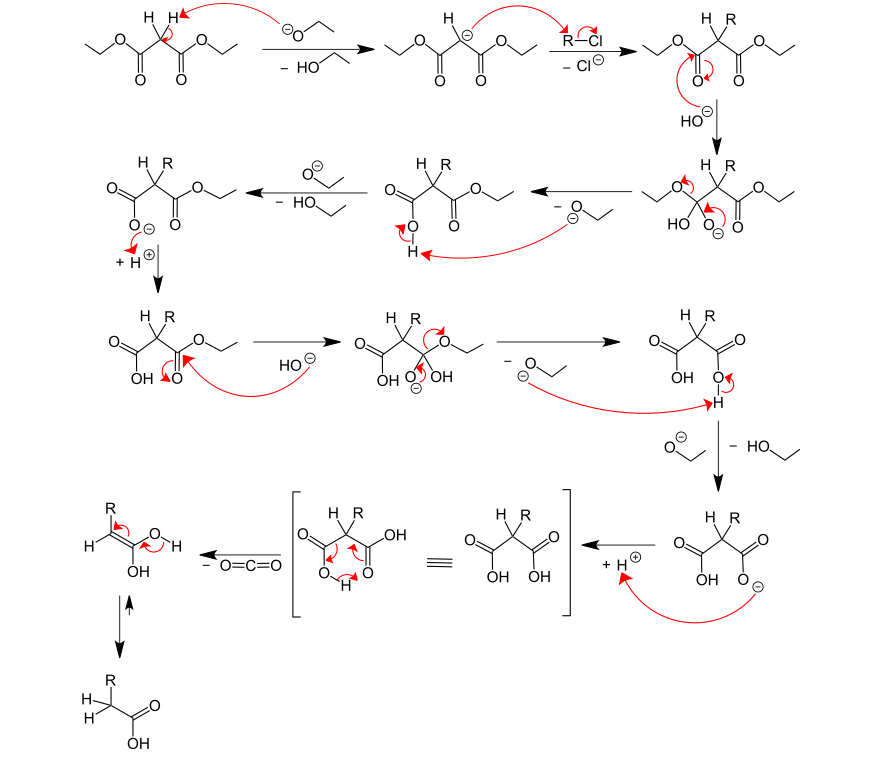
- Synthesis of an α-disubstituted carboxylic acid from the commonly used diethyl malonate . (X = Br, Cl or I, R = alkyl radical)
functionality
By flanking the α-carbon by two Estergruppen the bound there are hydrogen atoms particularly acidic (pK s = 13). Consequently, deprotonation is favored at this position. The ester groups can be hydrolyzed with base catalysis ( ester hydrolysis ). Because of the resulting carboxylic acid in the β-position, decarboxylation is greatly facilitated by heating and is further promoted by the acidic reaction conditions. The nucleophilic substitution of the haloalkane follows an S N 2 mechanism . Accordingly, branched alkyl radicals of the haloalkane are unfavorable for the reaction because of the steric hindrance . With regard to the nucleophilic properties of bases, a base should be used which results in the same ester when one of the carbonyl carbons is substituted. For example, for diethyl malonate, ethanolate .
Individual evidence
- ↑ Paula, Yurkanis, Bruice: Organic Chemistry. 4th edition, Prentice-Hall, 2003, ISBN 0-131-41010-5 , p. 821.
literature
- Paula, Yurkanis, Bruice: Organic Chemistry. 4th edition, Prentice-Hall, 2003, ISBN 0-131-41010-5 , pp. 821-822.
- KPC Vollhardt, Neil E. Schore, Organic Chemistry , Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, 4th edition, H. Butenschön, ISBN 3-527-31380-X , pp. 1228-1229.
