Threose
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
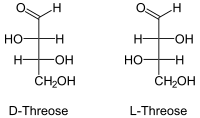
| Fischer projection , open-chain representation | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Threose | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 8 O 4 | ||||||||||||||||||
| Brief description |
syrupy, colorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 120.10 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Threose is a monosaccharide from the group of tetroses . There are two stereoisomers , the D- threose and the L- threose . Due to the terminal aldehyde group, they belong to the aldoses . The IUPAC name of the D form is (2 S , 3 R ) -2,3,4-trihydroxybutanal. L- threose [synonym: (2 R , 3 S ) -2,3,4-trihydroxybutanal] is of little importance. If “Threose” is mentioned in this text or in the scientific literature without any additional name ( prefix ), D- Threose is meant. The erythrose is a diastereomer of Threose.
The stereochemical configuration of threose gives its name to the descriptor threo - which is used as a semi-systematic addition to the name to identify molecules with similar configurations.
Reactions
The oxidation of threose, for example with nitric acid , produces tartaric acid . During the reduction, for example with sodium borohydride , threit is formed .
literature
- M. Steiger, T. Reichstein: Crystallized acetone-d-threose and a simple method for the production of d- and l-threose , in: Helvetica Chimica Acta , 1936 , 19 , pp. 1016-1019; doi : 10.1002 / hlca.193601901134 .
Individual evidence
- ↑ a b c data sheet D -Threose from Sigma-Aldrich , accessed on June 13, 2011 ( PDF ).