Erythrosis
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
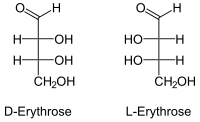
| Fischer projection , open-chain representation | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Erythrosis | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 8 O 4 | ||||||||||||||||||
| Brief description |
colorless, syrupy liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 120.10 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| solubility |
good in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Erythrose is a monosaccharide from the group of tetroses . Because of the terminal aldehyde group, erythrosis belongs to the aldoses . There are two stereoisomers , D -Erythrosis and L -Erythrosis . The IUPAC name of the D form is (2 R , 3 R ) -2,3,4-trihydroxybutanal. L- erythrosis [synonym: (2 S , 3 S ) -2,3,4-trihydroxybutanal] is of little importance. The Threose is a diastereomer to erythrose.
Erythrose was first isolated from rhubarb in 1849 by the French pharmacist Louis Feux Joseph Garot (1798–1869) and was so named because of its red color in the presence of alkalis (from ancient Greek ἐρυθρός, "red").
The stereochemical configuration of erythrosis gives its name to the descriptor erythro - which is used as a semi-systematic addition to the name to identify molecules with similar configurations. When "erythrosis" is mentioned in this text or in the scientific literature without any additional name ( prefix ), it means D -Erythrosis.
Reactions
Erythrose reacts slowly with hydrochloric acid to form lactic acid . In carbohydrate metabolism is erythrose 4-phosphate is an important intermediate.
Individual evidence
- ↑ a b entry on erythosis. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ↑ a b Erythrose data sheet from Sigma-Aldrich , accessed on June 12, 2011 ( PDF ).
- ↑ Obituary of Garot (1869) Journal de pharmacie et de chimie, 4th series, 9 : 472-473.
- ↑ Garot (1850) [1] (On the red coloring material of exotic and indigenous rhubarb and on its application (as a coloring material) in the arts and in pharmacy), Journal de Pharmacie et de Chimie, 3rd series, 17: 5 -19. Erythrose is named on p. 10: "Celui que je propose, sans y attacher toutefois la moindre importance, est celui d'érythrose, du verbe grec 'ερυθραινω, rougir (1)." (The one [ie, name] that I propose, without attaching any importance to it, is that of erythrose, from the Greek verb ερυθραινω, to redden (1).
- ^ Hermann PT Ammon (ed.): Hunnius pharmaceutical dictionary. 9th, revised and expanded edition. Walter de Gruyter, Berlin et al. 2004, ISBN 3-11-017475-8 .