Sulfolane
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
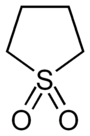
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Sulfolane | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 8 O 2 S | ||||||||||||||||||
| Brief description |
white, liquid, odorless mass |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 120.17 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.261 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
27.4 ° C |
||||||||||||||||||
| boiling point |
285 ° C |
||||||||||||||||||
| Vapor pressure |
3.5 Pa (30 ° C) |
||||||||||||||||||
| solubility |
very light in water (1266 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Sulfolane , systematic name tetrahydrothiophene-1,1-dioxide , is a sulfone and is a polar solvent . The molecule can be described as a five-membered ring made up of four carbon atoms and a double-oxidized sulfur atom.
properties
The flash point is 165 ° C, the specific heat capacity is 1.5 J / (g · K). Sulfolane is assigned to the water hazard class WGK 1.
use
In technology it is used as a solvent , e.g. B. for the extraction of aromatics . It is used in gas cleaning to separate sulfur compounds.
Unlike dimethyl sulfoxide or dimethyl sulfone , it cannot be used medicinally to transport substances (e.g. drugs ) through cell membranes as a solvent , since it is itself relatively toxic and its ability to transport biomembrane is poor.
Individual evidence
- ↑ a b c d e f g h Entry on tetrahydrothiophene-1,1-dioxide in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ Entry on Tetrahydrothiophene 1,1-dioxide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ ( page no longer available , search in web archives: Sulfolane ) at www.sulfolane.com.

