Skatole
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
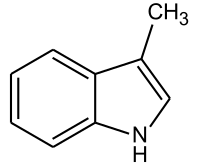
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Skatole | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 9 N | |||||||||||||||
| Brief description |
white to brownish crystals with an unpleasant odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 131.17 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
95 ° C |
|||||||||||||||
| boiling point |
265-266 ° C |
|||||||||||||||
| solubility |
very bad in water (450 mg l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
The skatole (from Greek σκῶρ , Gen. σκατός "droppings, manure") is a very intense and unpleasant smelling indole compound . The smell of feces is mainly due to skatole.
Occurrence
Skatole occurs in human and animal feces , in manure (up to 0.1%), in the secretion of the civet and in many plants as a component of the flower scent. The meat of uncastrated boars also contains skatole.
Emergence
Meat consists of proteins , the building blocks of proteins are the amino acids . One of these amino acids is tryptophan , an essential amino acid that cannot be produced by the human organism itself, but must be ingested with food . The conversion of amino acids, for example during digestion, into various breakdown products that are partially absorbed and partially excreted by the body, is done by enzymes. Along with indole, skatole is one of the breakdown products of tryptophan. The relationship between tryptophan, indole and skatole can be recognized by the ring structure that all three have in common. Since tryptophan is particularly common in animal proteins (including muscle meat), skatole is present in the stool in a correspondingly larger amount when meat is consumed frequently.
Presentation and extraction
Skatole can be chemically made from indole. However, since direct methylation of indole would yield 1-methylindole (syn .: N -methylindole), the N – H group must first be protected with a protective group . There are z. B. Trialkylsilyl protecting groups are preferred, since they are resistant to the subsequent deprotonation (or lithiation) with alkyllithium compounds. After reaction with methyl iodide , the protective group is removed again and skatole is obtained.
properties
Chemical properties
Skatole forms white crystals. It reacts violently with strong oxidizing agents , strong acids , acid chlorides and acid anhydrides .
Physiological properties
The odor threshold for skatole in air is extremely low in humans. In the literature, values of 1.5 µg · m −3 , 0.0005 to 6.4 µg · m −3 , and 0.0004 µg · m −3 can be found . In water the odor threshold is 10 µg · l −1 , in sunflower oil 15.6 ppb .
use
In high dilution, Skatol has a pure, low-odor odor of roses and traces of it are used in perfumery.
Analytics
The detection of skatole can in simple investigation goods by means of headspace - gas chromatography done. In more complex test materials, after adequate sample preparation, the qualitative and quantitative determination of skatole can be carried out by coupling gas chromatography with mass spectrometry . The coupling of UHPLC with mass spectrometry is also used for more complex specimens .
Individual evidence
- ↑ a b c d e Data sheet Skatol (PDF) from Carl Roth , accessed on December 14, 2010.
- ↑ a b Datasheet Skatole at Sigma-Aldrich , accessed on April 23, 2011 ( PDF ).
- ^ Hans Beyer , Wolfgang Walter : Organic chemistry. S. Hirzel Verlag, Stuttgart 1984, ISBN 3-7776-0406-2 , p. 727.
- ^ ValentinHarter: Comparative observation of behavior in male surgically castrated, immunocastrated and uncastrated fattening pigs. Dissertation, LMU Munich, 2017, pp. 3–4.
- ^ Lebensministerium.at: State of the art in composting - basic study . September 29, 2005, p. 68.
-
↑ Dorothea Lösel: Attempts to improve the sensory meat quality in pigs by nutritive inhibition of the skatole formation University of Hohenheim, dissertation, 2006, p. 3.
According to: JA Zahn, AA DiSpirito u. a .: Correlation of human olfactory responses to airborne concentrations of malodorous volatile organic compounds emitted from swine effluent. In: Journal of environmental quality. Volume 30, Number 2, 2001 Mar-Apr, pp. 624-634, PMID 11285926 . - ↑ NIIR Board: Food Colors, Flavors and Additives Technology Handbook. Niir Project Consultancy Services, 2004, ISBN 978-8-186-62376-3 , p. 287 ( limited preview in Google book search).
- ↑ Hans-Dieter Belitz: Textbook of food chemistry. Springer-Verlag, 2013, ISBN 978-3-662-08308-6 , p. 276 ( limited preview in the Google book search).
- ↑ Gerhard Eisenbrand: RÖMPP Lexikon Lebensmittelchemie, 2nd edition, 2006. Georg Thieme Verlag, 2014, ISBN 978-3-131-79282-2 , p. 1076 ( limited preview in the Google book search).
- ↑ Parfumo List of Perfumes Containing Skatole , accessed April 27, 2017.
- ^ Franz von Bruchhausen: Hager's Handbook of Pharmaceutical Practice . ISBN 3-540-52688-9 , pp. 182 ( limited preview in Google Book search).
- ↑ K. Verplanken, J. Wauters, J. Van Durme, D. Claus, J. Vercammen, S. De Saeger, L. Vanhaecke: Rapid method for the simultaneous detection of boar taint compounds by means of solid phase microextraction coupled to gas chromatography / mass spectrometry. In: J Chromatogr A. 1462, Sep 2, 2016, pp. 124-133. PMID 27492596
- ↑ Y. Zhou, SA Hallis, T. Vitko, IH Suffet: Identification, quantification and treatment of fecal odors released into the air at two wastewater treatment plants. In: J Environ Manage. 180, Sep 15, 2016, pp. 257-263. PMID 27235805
- ^ MA Mahmoud, A. Buettner: Characterization of aroma-active and off-odor compounds in German rainbow trout (Oncorhynchus mykiss). Part I: Case of aquaculture water from earthen-ponds farming. In: Food Chem. 210, Nov 1, 2016, pp. 623-630. PMID 27211690
- ↑ K. Verplanken, J. Wauters, V. Vercruysse, M. Aluwé, L. Vanhaecke: Development and validation of a UHPLC-HR-Orbitrap-MS method for the simultaneous determination of androstenone, skatole and indole in porcine meat and meat products . In: Food Chem. 190, Jan 1, 2016, pp. 944-951. PMID 26213060

