Oxepan
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Oxepan | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 12 O | |||||||||||||||
| Brief description |
clear, slightly yellowish liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 100.16 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.90 g cm −3 |
|||||||||||||||
| boiling point |
124-126 ° C |
|||||||||||||||
| Refractive index |
1.4400 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Oxepan is a chemical compound from the group of saturated heterocycles . It is the simplest seven - membered oxygen - containing heteroaliphatic.
presentation
Oxepane can be prepared from the cyclization of 1,6-hexanediol in DMSO at 190 ° C., but only in poor yield. Another possibility, which, however, gives a comparably poor yield, is the cyclization of 1,6-dichlorohexane with potassium hydroxide .
Reactions
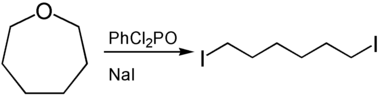
Oxepanes can be used to prepare α, ω-functionalized derivatives of hexane by ring opening . Lewis or Brønsted acids can be used to open the ring . The reaction of Oxepan with phenyl dichlorophosphate and sodium iodide produces 1,6-diiodohexane.
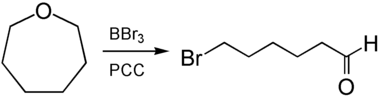
6-Bromohexanal is accessible from the reaction with boron tribromide with subsequent oxidation by PCC .
Individual evidence
- ↑ a b c Entry on Hexamethylene Oxide at TCI Europe, accessed on October 31, 2016.
- ↑ A. Müller, W. Vanc, in: Monatsh. Chem. , 1947 , 77 , pp. 259-263.
- ↑ A. Misono, T. Osa, Y. Sanami, in: Bull. Chem. Soc. Jpn. , 1968 , 41 , pp. 2447-2453.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-408.
- ↑ VJ Traynelis, WL Hergenrother, HT Hanson, JA Valicenti, in: J. Org. Chem. , 1964 , 29 , pp. 123-129.
- ↑ HA Zahalka, Y. Sasson, in: Synthesis , 1986 , 9 , pp. 763-765.
- ↑ H.-J. Liu, LM Shewchuk, M. Llinas-Brunet, in: Heterocycles , 1986 , 24 , pp. 3043-3046.
- ↑ SU Kulkarni, U. Surendra, VD Patil, in: Heterocycles , 1982 , 18 , pp. 163-167.