Acylation
Under acylation refers to the introduction of an acyl group in an existing chemical compound . Carboxylic acids can serve as acylating agents, but the more reactive carboxylic acid halides are often used, which react with an electron-rich molecule after activation .
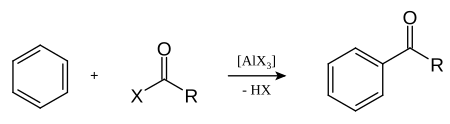
Friedel-Crafts acylation is an important acylation of aromatic systems . It uses aluminum halides as Lewis acids to form the acyl group .
Intramolecular acylations are also known, including the Fries rearrangement . Here, the acyl group is split off from a phenyl ester by a Lewis acid and then attaches itself again to the ring.
More broadly, the introduction of an aldehyde function ( formylation ) can be viewed as a special case of acylation. The formylations include hydroformylation , Vilsmeier formylation, and Gattermann-Koch and Reimer-Tiemann reactions .
However, acylations are not limited to the formation of carbon-carbon bonds. The formation of amides from amines and esters from alcohols can also be referred to as acylation , especially in a biochemical context. In biological systems, by acylation can transacylases , a subset of the transferases , enzymatically be performed. In this context, the transpeptidases for the construction of peptides are also important .
literature
- Entry to acylation. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- Entry to acyl. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- Entry on transacylases. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.