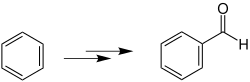
Gattermann synthesis
The Gattermann synthesis is a name reaction in organic chemistry that is used to produce aromatic aldehydes from phenols or other aromatics . It was discovered in 1906 by the Goslar chemist Ludwig Gattermann (1860–1920) and is also known as Gattermann aldehyde synthesis . This variant of Friedel-Crafts acylation can also be used to synthesize individual hydrocarbon compounds , heterocycles such as furan , pyrrole and indole derivatives and thiophene .
Overview reaction
In the Gattermann reaction, aromatics (except for aromatic amines and nitro compounds ) react with hydrocyanic acid and hydrogen chloride in the presence of the catalyst zinc chloride (ZnCl 2 ) or aluminum chloride (AlCl 3 ) to form aromatic aldehydes.
mechanism
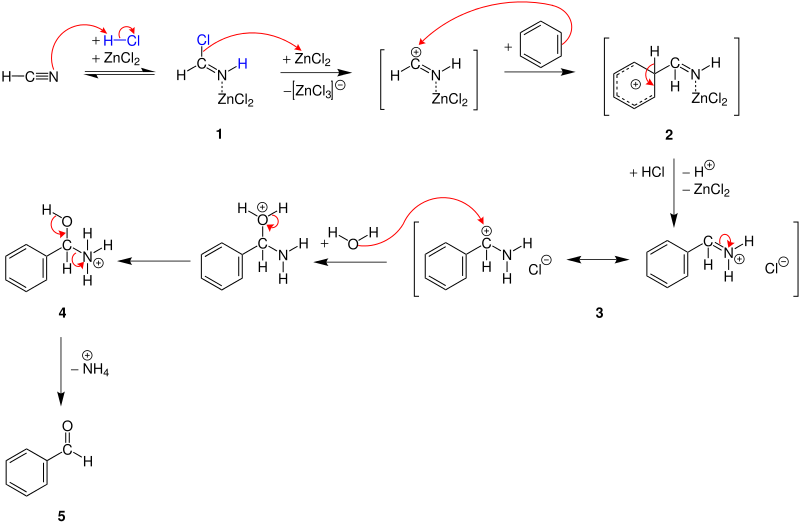
The Gattermann reaction is an electrophilic aromatic substitution . A possible mechanism is shown using the example of hydrogen cyanide , hydrogen chloride and benzene, which react to form benzaldehyde ( 5 ). Zinc chloride is used as a catalyst . In the first step, the lone pair of electrons in nitrogen attacks the hydrogen atom in hydrogen chloride. A form of imide chloride is formed , which reacts with the catalyst to form complex 1 . Further addition of zinc chloride produces a carbenium ion , which is the electrophile for electrophilic aromatic substitution as the next step. The carbenium ion and the benzene interact, so that a mesomerism-stabilized σ-complex 2 is formed. After the subsequent deprotonation , compound 2 is rearomatized and converted to an iminium salt 3 by adding hydrogen chloride . The next step is hydrolysis and subsequent proton migration , so that an α-amino alcohol 4 is formed. The charge on the nitrogen atom in 4 polarizes the CN bond, so that the carbon atom receives a partial positive charge and thus polarizes the OH bond. As a result, a proton and an ammonia molecule are split off and react to form an ammonium ion . The product is benzaldehyde ( 5 ).
If polyhydric phenols or phenol ethers are used as the starting material, no catalyst is required.
variants
Gattermann-Adams reaction
The Gattermann-Adams reaction is also a name reaction in organic chemistry. During the reaction, the hydrocyanic acid is released from zinc cyanide under the action of hydrogen chloride and can be converted as in the Gattermann reaction. The activity of the resulting zinc (II) chloride is sufficient to act as a catalyst in the reaction with more reactive phenols. When reacting with inert phenols, aluminum chloride must also be added as a catalyst.
Gattermann-Koch reaction
The Gattermann-Koch synthesis is also one of the name reactions in organic chemistry, which was named after the German chemist Ludwig Gattermann (1860–1920) and the German-American chemist Julius Arnold Koch (1864–1956). Here aromatics react with carbon monoxide and hydrogen chloride and the catalysts aluminum chloride or copper (I) chloride to form formylated aromatics.
mechanism
The Gattermann-Koch reaction is, like the Gattermann synthesis, an electrophilic aromatic substitution . The starting materials carbon monoxide , hydrogen chloride and the catalyst aluminum chloride react according to a possible mechanism to form an acylium ion 1 , which the aromatic attacks with the formation of a hexadienyl cation 2 . The cation 2 is deprotonated and so rearomatized, whereby benzaldehyde ( 3 ) is formed with elimination of aluminum chloride .
The Gattermann-Koch synthesis must take place with the exclusion of water . Phenols, phenol ethers and nitrobenzenes are not suitable for this variant and therefore do not react.
See also
- Becker, Heinz GO: Organikum , 22nd edition, Wiley-VCH, Weinheim, 2004, ISBN 3-527-31148-3 .
- Hans Beyer , Wolfgang Walter : Textbook of Organic Chemistry ; S. Hirzel Verlag, Stuttgart - Leipzig 1998, 23rd revised. and updated edition; ISBN 3-7776-0808-4 .
- Organikum , VEB Deutscher Verlag der Wissenschaften, Berlin 1976.
Individual evidence
- ^ A b Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, ISBN 3-342-00280-8 , p. 355.
- ↑ T. Laue, A. PLagens: Name and catchword reactions of organic chemistry . Teubner Verlag, 2006, ISBN 3-8351-0091-2 , p. 149-152 .
- ^ BP Mundy, MG Ellert, FG Favaloro, Jr .: Name Reactions in Organic Synthesis . 2nd Edition. Wiley & Sons, 2005, ISBN 0-471-22854-0 , pp. 272-273 .
- ^ László Kürti , Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis - Background and Detailed Mechanisms . Elsevier Inc., 2005, ISBN 978-0-12-369483-6 , pp. 184-185 .
- ^ R. Adams, I. Levine: Simplification of the Gattermann Synthesis of Hydroxy Aldehydes . In: J. Am. Chem. Soc. tape 45 , 1923, pp. 2373-2377 , doi : 10.1021 / ja01663a020 .
- ↑ Gattermann, L .; Koch, JA: A Synthesis of Aromatic Aldehydes . In: Ber. tape 30 , 1897, pp. 1622 , doi : 10.1002 / cber.18970300288 .
- ↑ T. Laue, A. PLagens: Name and catchword reactions of organic chemistry . Teubner Verlag, 2006, ISBN 3-8351-0091-2 , p. 149-152 .
- ^ László Kürti , Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis - Background and Detailed Mechanisms . Elsevier Inc., 2005, ISBN 978-0-12-369483-6 , pp. 184-185 .