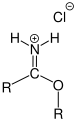
Iminium ion
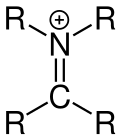
Iminium ions , also iminium cations, are mesomeric stabilized organic cations with a bond between a nitrogen atom and a carbon atom.
Reactions in which iminium ions are formed as intermediates (selection)
- Beckmann rearrangement
- Duff reaction
- Fragmentation of ionized amines via α-cleavage in the mass spectrometer
- Leuckart-Wallach reaction
- Mannich reaction
- Pinner reaction
- Pictet-Spengler reaction
- Ugi reaction
- Vilsmeier-Haack reaction
Production and occurrence
Iminium ions arise as an intermediate z. B. in the dehydration of hemi-aminals . One often speaks of a “protonated imine” when talking about an iminium ion. This name comes from the fact that imines become iminium ions through protonation. The iminium ion is subject to a mesomerism, which affects its reactivity. A mesomeric limit form is then a carbenium ion. Chloride salts of N- acylated iminium ions are formed when imines are reacted with carboxylic acid chlorides .

Examples
Since the iminium ion can have very different forms, some examples will now be given here to illustrate the variety of possibilities.
Chlorine iminium ion intermediate in the Vilsmeier-Haack reaction
Iminium ion intermediate in the Pictet-Spengler reaction
Iminium ion intermediate in the Mannich reaction
, Also called Iminumchloridsalz Pinner salt as in the Pinner reaction occurs
See also
Individual evidence
- ↑ Joachim Buddrus: Fundamentals of Organic Chemistry , 4th Edition, de Gruyter Verlag, Berlin, 2011, pp. 40–41, ISBN 978-3-11-024894-4 .
- ↑ a b c K. PC Vollhardt , NE Schore: Organische Chemie , 4th edition, Wiley-VCH, Weinheim 2005 , ISBN 978-3-527-31380-8 , p. 879.
- ↑ W. Schwarze, K. Drauz, J. Martens: Reaction of 3-thiazolines with carboxylic acid chlorides , Chem.-Ztg. 1987, 111, pp. 149-153.