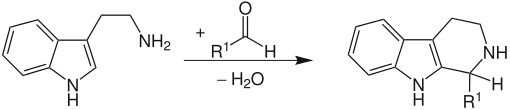
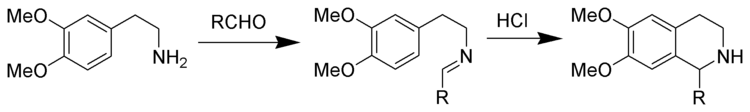
Pictet-Spengler reaction
The Pictet-Spengler reaction is a chemical reaction for the production of heterocycles . β-Arylethylamines, such as tryptamine , cyclize with an aldehyde, with elimination of water . The reaction is usually acid-catalyzed and carried out in the heat. Particularly reactive aromatics give good yields even under physiological conditions. The Pictet-Spengler reaction is a special case of the Mannich reaction .
The reaction was described in 1911 by Amé Pictet (1857–1937) and Theodor Spengler (1886–1965) and has developed into an important reaction for the synthesis of alkaloids and active pharmaceutical ingredients .
Particularly nucleophilic β-arylethylamines such as. B. in indole derivatives or in pyrrole derivatives give the ring closure products in good yields under mild conditions. Little nucleophilic aryl radicals such as. B. a phenyl radical in β-phenethylamine, however, lead to poor yields or require high temperatures and strong acids . The reaction published by Pictet and Spengler was carried out with β- phenethylamine and the formaldehyde dimethylacetal with hydrochloric acid and the product obtained was tetrahydroisoquinoline .
Like the Mannich reaction, the less reactive ketones either give only low yields or do not undergo any desired reaction at all.
The Pictet-Spengler reaction has been successfully used in solid phase synthesis in combinatorial chemistry . The analogous reaction with aryl-β-ethanol (instead of the amine) is called the Oxa-Pictet-Spengler reaction .
Reaction mechanism
The aldehyde initially reacts with the amine. After protonation and subsequent elimination of water, an iminium ion is formed . The driving force of the reaction is the electrophilicity of the iminium ion, which can now be attacked by the more electron-rich, aromatic ring. After splitting off a proton (with rearomatization ), the heterocycle is obtained. The reaction is a 6-endo-trig reaction which is preferred according to Baldwin's rules .
variants
Tetrahydroisoquinoline synthesis according to Pictet-Spengler
Tetrahydroisoquinolines can be synthesized according to the principle of the Pictet-Spengler reaction. The reaction conditions are comparatively drastic, higher temperatures and stronger acids such as hydrochloric acid , trifluoroacetic acid or even super acids are required.
N- acyliminium variant of the Pictet-Spengler reaction
Inactive aromatics can be made to react under mild conditions. This takes advantage of the fact that an N -acyliumiminium ion is an excellent electrophile. The amine is activated by reacting an acylating agent (e.g. an acid chloride or anhydride) and the reaction takes place via the N- acyliumiminium ion as a reactive intermediate.
Tadalafil was made via the N- acyliminium Pictet-Spengler reaction.
See also
Individual evidence
- ↑ A. Pictet, T. Spengler: In About the formation of isoquinoline derivatives through the action of methylal on phenyl-ethylamine, phenyl-alanine and tyrosine Chem. Ber. 1911 , 44 , 2030-2036.
- ↑ WM Whaley, TR Govindachari: In The Pictet-Spengler synthesis of tetrahydroisoquinolines and related compounds Org. React. 1951 , 6 , 74.
- ↑ G. Hahn, H. Ludewig: In synthesis of tetrahydro-harmane derivatives under physiological conditions Chem. Ber. 1934 , 2033.
- ↑ TE Nielsen, F. Diness, M. Meldal: In Solid-Phase Synthesis of Pyrroloisoquinolines via the Intramolecular N-Acyliminium Pictet-Spengler Reaction Curr. Opin. Drug Discov. Devel. 2003 , 6 , 801-814.
- ↑ TE Nielsen, M. Meldal: In Solid-Phase Synthesis of Pyrroloisoquinolines via the Intramolecular N-Acyliminium Pictet-Spengler Reaction J Comb Chem . 2005 , 7 , 599-610.
- ^ Cox, Cook: In The Pictet-Spengler condensation: a new direction for an old reaction Chemical Reviews 1995 , 95 , 1800-1802.
- ^ A. Yokoyama: In Prototype Pictet-Spengler Reactions Catalyzed by Superacids. Involvement of Dicationic Superelectrophiles J. Org. Chem. 1999 , 64 , 611-617.
- ↑ BE Maryanoff, H.-C. Zhang, JH Cohen, IJ Turchi, CA Maryanoff: In Cyclizations of N-acyliminium ions Chem. Rev. 2004 . 104 , 1431-1628.
- ↑ D. Bonnet, A. Ganesan: In Solid-Phase Synthesis of Tetrahydro-β-carbolinehydantoins via the N-Acyliminium Pictet-Spengler Reaction and Cyclative Cleavage J. Comb. Chem. 2002 , 4 , 546-548.